Audit Management Simplified
AmpleLogic Audit Management Software is a comprehensive solution designed to streamline and enhance the audit processes for organizations operating in regulated industries such as pharmaceuticals, biotech, medical devices, and others. This software offers a range of features and benefits tailored to meet the specific needs and challenges of GMP (Good Manufacturing Practice) audits and compliance requirements.
With AmpleLogic Audit Management Software, organizations can automate and simplify various aspects of the audit lifecycle, from planning and scheduling to execution, reporting, and follow-up actions. By leveraging a low-code platform, this software ensures flexibility and customization to adapt to the unique workflows and requirements of each organization.
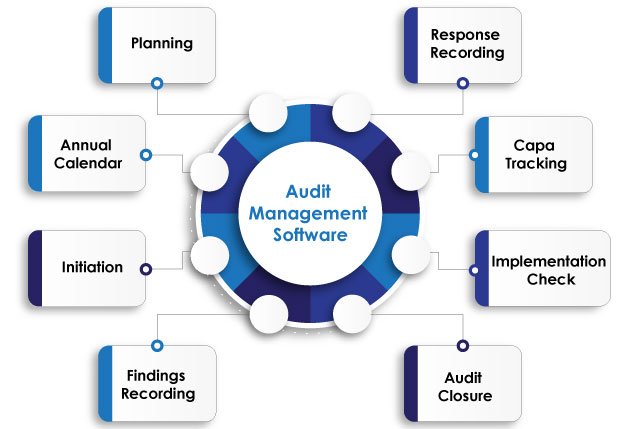
Simplifying Audit Processes for Enhanced Efficiency
Audit Planning & Scheduling
Easy calendar management and schedule notifications.
Flawless Execution
Secure data capture and comprehensive checklist management.
CAPA Integration
Seamlessly link audit findings with corrective actions.
Graphical Reports
Intuitive insights for informed decision-making.
Centralized Access
Secure repository for all audit-related information.
Customizable & Compliant
Tailored to industry standards and regulatory requirements.
Benefits of AmpleLogic Audit Management
Streamlined audit processes reduce manual effort, saving time and resources.
Ensures adherence to regulatory standards and GMP guidelines, reducing the risk of non-compliance penalties.
Centralized access to audit data promotes transparency and accountability across the organization.
Automated data capture minimizes errors and ensures the accuracy of audit information.
Intuitive graphical reports provide actionable insights, enabling informed decision-making at all levels.
Facilitates the identification of trends and areas for improvement, driving ongoing quality enhancement efforts.
Robust data security measures safeguard sensitive audit information, ensuring confidentiality and compliance with data protection regulations.
Adaptable to organizations of varying sizes and complexities, accommodating growth and changing needs over time.
Reduces the need for manual processes and paper-based documentation, resulting in cost savings over the long term.
Ensures product quality and reliability, enhancing customer trust and satisfaction.
Frequently Asked Questions
Can AmpleLogic integrate with other quality modules?
Yes, it seamlessly integrates with CAPA management and other quality modules for consistent advancement.
How secure is the data capture in AmpleLogic Audit Management?
Data capture is highly secure, ensuring confidentiality and compliance with electronic record standards.
What industries can benefit from AmpleLogic Audit Management?
Pharmaceutical, biologics, medical devices, and various manufacturing sectors can leverage its capabilities.
Is training required to use AmpleLogic Audit Management?
Minimal training is needed due to its intuitive interface and user-friendly design.
Can AmpleLogic Audit Management software generate custom reports?
Yes, it provides graphical reports tailored to specific needs, offering actionable insights for management.
Contact us
Your Pharma Automation Starts Here
We’re here to address your inquiries and assist you in identifying the solutions that best align with your requirements. Here’s why choosing us is your strategic advantage:
Your benefits:
- Client-oriented
- Independent
- Competent
- Results-driven
- Problem-solving
- Transparent
What happens next?
1
Schedule a call at your convenience
2
Discovery and consultation session
3
Get your custom proposal