Transformative Change Management Solution
AmpleLogic Change Control is a cutting-edge software solution tailored for GMP-driven industries, enabling seamless automation, compliance assurance, and enhanced productivity throughout the change management lifecycle. From initiation to closure, revolutionize your change processes with AmpleLogic’s intuitive platform.
Efficiency Amplified: Key Features
Unified System Integration
Automate processes with seamless integration across departments.
Transparent Workflow Management
Ensure clarity and traceability throughout the change process.
Comprehensive Initiation and Tracking
Effortlessly manage change requests from inception to completion.
Seamless Integration
Integrate with CAPA, LMS, and DMS for holistic quality management.
Robust Analytics and Reporting
Gain actionable insights with advanced analytics and reporting features.
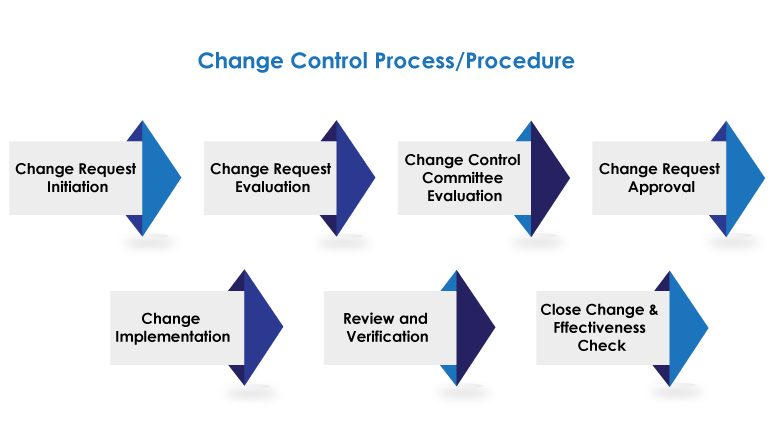
Navigating Change with Precision
Efficiently launch change requests and obtain necessary approvals, ensuring a smooth start to the change process.
Thoroughly assess proposed changes, evaluating their impact and feasibility before proceeding to implementation.
Execute approved changes with precision, verifying their successful implementation and compliance with regulatory standards.
Continuously monitor the progress of change initiatives, generating comprehensive reports for transparency and accountability.
Formally close change requests, documenting all relevant information and ensuring proper closure of the change control process.
Frequently Asked Questions
What industries can benefit from AmpleLogic Change Control?
AmpleLogic Change Control is tailored for industries such as pharmaceuticals, biotechnology, medical devices, and more.
Is AmpleLogic Change Control compliant with regulatory standards?
Yes, our solution adheres to regulatory requirements such as 21 CFR Part 11 and EU Annex 11.
Can AmpleLogic Change Control integrate with existing systems?
Absolutely, our platform seamlessly integrates with other QMS modules for enhanced functionality.
How customizable is AmpleLogic Change Control?
Our solution is highly customizable to suit the unique needs and workflows of your organization.
What support options are available for AmpleLogic Change Control users?
We offer comprehensive support services and consultations to ensure a smooth implementation and ongoing usage.
Contact us
Your Pharma Automation Starts Here
We’re here to address your inquiries and assist you in identifying the solutions that best align with your requirements. Here’s why choosing us is your strategic advantage:
Your benefits:
- Client-oriented
- Independent
- Competent
- Results-driven
- Problem-solving
- Transparent
What happens next?
1
Schedule a call at your convenience
2
Discovery and consultation session
3
Get your custom proposal