A long list of data integrity warnings received by pharma companies has made them train their focus on building and fortifying their tracking mechanisms to prevent things from going wrong. One of the solutions is digitization and building a data repository to make it easily available to analyze issues and predict challenges.
However, the current challenge is that the digital transformation is going slower than expected, resulting in the engagement of quality resources for a longer time. Implementation of pre-validated software’s like LIMS, DMS, QMS, LMS are taking longer than 12 months and electronic batch manufacturing records (eBMR) are taking more than three years. Most implementations are going beyond the scheduled time due to rework and mid-way requirement changes.
This impacts all the documents and hence requires repeated updations with the latest changes. These complications should be addressed with smooth and flexible methods. In general, the OQ duration is 60 percent of the total effort of a project. It is questionable whether such an effort is constructive for configurable software packages (GAMP category 4 & 5). Even after investing a good amount of money and resources, they are not able to realize the expected outcomes.
Key challenges
On the pharma industry front
- Business user requirements are not documented properly
- Unstable senior management.
- Functional requirements are not detailed enough and do not have traceability to user requirements
- Very expensive change request. (Cost of change)
- Traceability matrix is not maintained or updated
On the supplier front
- Rigid systems (Need to write code for every major change)
- Lack of domain knowledge and unskilled programmers
- Traditional software implementation methodologies
V Model: Verification and Validation Model
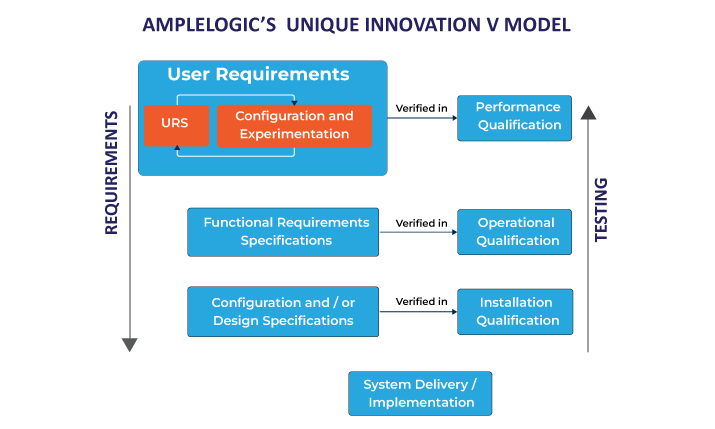
Most of the software implementations in the pharma industry will follow this model. The success of the V Model is when requirements are well defined with no ambiguity and acceptance criteria well defined.
In the V Model, there are long cycle times from user requirement specification to user acceptance test and requirements may change in the meantime. The modeling of user requirements without seeing a running piece of software is abstract and usually, requirements for modification arise when the final user deals with the running software for the first time.
The traditional V model software implementation methodologies will not create an interest among the business teams as it takes longer to address a change. If any changes happen midway, then the test documents, along with requirement documents, have to be updated. The cost of major change/ requirement gaps realized in the OQ stage will turn out to be very expensive and delays the validation process.
From the past 10 years, regulatory inspections focus more on software and computer system validation. Deviations have been cited across all steps of computer validation from writing specification and risk assessment to IQ/OQ/PQ, revalidation, reporting, and change control.
The traditional V Model: It must be revisited based on our real-time experiences
One important aspect is the Configuration and Experimentation phase. The introduction of this phase in between the User Requirement Specification and Functional Requirement Specification of the V model will help the business users in understanding the pre-validated software and relating the software with his problem statement.
This approach helps in realizing the requirements before the finalization of functional requirement specifications. The business user’s clear on the software outcome.
Adding the Configuration and Experimentation phase in the implementation cycle will address regulatory audit observations related to revalidation, deviations and multiple release management.
The Configuration and Experimentation approach cannot be achieved through traditional configurable software. The configurable Software must have visual modeling capabilities which are commonly known as No code/Low Code Development Platforms. These platforms support Visual Modelling (The user can see the making of application)
The organizations must ensure that the software service provider agrees to offer configuration and experimentation as part of the V Model. If the pre-validated software or configured software follows this model, implementation times can be reduced by 70 percent.
This way, gaps can be easily identified during the Configuration and Experimentation phase and as implementation time gets reduced and allows us to complete OQ in 25 percent of the total project time.
No code/ Low code Platform
These platforms allow business and IT to collaborate in real-time, using visual models to capture business requirements as well as quickly iterate and scale apps while ensuring nothing gets lost in translation. The platform allows users to quickly turn their ideas into building up applications and transforming their manual processes to digital within days.
Low Code or No-code development platform provides drag-and-drop tools that allow business process engineering (BPE) teams to develop software quickly without coding. The platforms provide drag-and-drop components to quickly assemble and design applications at reduced timelines and efforts. They also help increase business productivity and efficiency at the work levels. Both developers and non-developers can use these tools to practice rapid application development with customized workflows and functionality.
Global enterprises are looking for No Code Platforms to build actionable digital strategies for every part of their business.
When prevalidated GMP software like QMS, DMS, LMS, eBMR and Batch Issuance software were built on using this No Code/ Low code Platforms then addressing the Requirement gaps or change will be faster and minimal time with less no of people involvement. Even change management becomes easy using No Code Platform. Processes can be changed every now and then, even for a small change in the traditional approach will take months’ time, using the No-code platform can happen in days’ time.
The platforms offer
- Visual modeling of business logic and workflows, with the ability to extend with custom code
- Visual definition of data models
- Drag-and-drop implementation of modern user interfaces for multiple devices
- Application change and life-cycle management
The way forward for pharma organizations
This is the time for pharma organizations to step towards selecting pre-validated software with visual modeling capabilities (No/ Low Code Platforms) that helps business users in appealing digital experiences with human readable application models by keeping the cost constant.
Way forward for IT organizations
Software suppliers must focus on increasing speed with visual modeling instead of focusing on the documentation during finalization of requirements. IT wings of pharma companies/ IT organizations should invest in No/ Low code development platforms, limiting the communication by keeping fewer resources and more productivity. The adoption of these platforms that are designed to empower application development professionals in IT to accelerate app delivery for more architecturally complex applications.