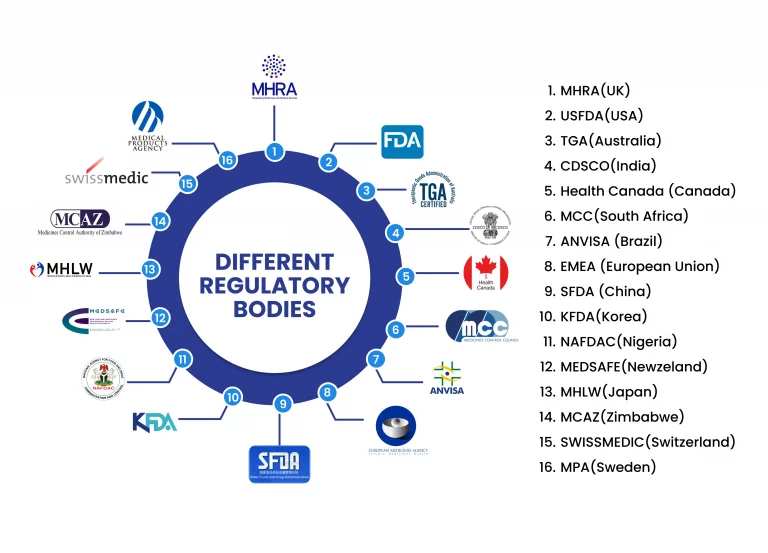
The pharmaceutical industry operates in a highly regulated and complex environment, facing numerous challenges that can impact productivity, compliance, and overall efficiency. Managing documents from several sources becomes challenging and time-consuming. However, eDMS Software is a power-packed electronic document management system exclusively designed for Pharmaceutical and Biotech companies, developed, and implemented exclusively to meet the cGMP needs of the Pharma domain with 21 CFR Part 11 compliance, EU Annex 11 compliance can easily eliminate all the challenges faced by different companies. Below are the few regulatory bodies that offer guidelines for generating, handling, and maintaining the journey of a drug development process.
However, the electronic document management system (eDMS) has become a game changer in today’s era. It is vital for the smooth functioning of the pharma operation in today’s complex and collaborative work environment. Simultaneously, eDMS addresses the challenges faced by the pharma industries in the recent scenario. In this article, we deep-dive into some of the most common issues faced by the pharma industries.
eDMS paves path for transformative change
In recent years, the pharmaceutical industry has witnessed a significant transformation driven by advancements in technology. This digital solution has been instrumental in streamlining processes, improving efficiency, and ensuring compliance across the pharmaceutical sector. No doubt it has become a game-changer by enhancing document control, improving regulatory compliance, enabling efficient information retrieval, ensuring data security, and streamlining audits. As a result, eDMS empowers pharma companies to operate more effectively in a highly regulated environment and like SOPs, Protocols, Specifications Annexures and more.
As the industry continues to evolve, eDMS will continue to play a crucial role in supporting innovation, collaboration, and overall success in the pharmaceutical sector. Moreover, the eDMS can be integrated with pharma electronic quality management software and digital learning management systems due to its robust document control capabilities, designed to manage documents more effectively and efficiently while ensuring compliance with all the applicable regulatory requirements. Also, the Software can incorporate various document formats.
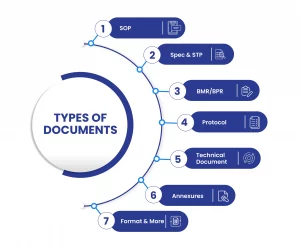
Types of documents that can be uploaded in the DMS Software
Key Pharmaceutical Industry Challenges & Solutions of eDMS Software
eDMS has come a long way and overtaken paper-based traditional systems as part of the shift towards greater transparency, making data increasingly searchable, accurate, easy data sort, store, retrieve, archive, and difficult to forge. Let us now have look at how eDMS solves the issues faced by the pharma industries:
1. Document Accessibility from Tab, Mobile, or Web Browser: eDMS platforms are designed to provide seamless access to documents from various devices, including tablets, mobile phones, and web browsers. With responsive web interfaces or dedicated mobile applications, authorized users can conveniently retrieve, review, and collaborate on documents regardless of their location or the device they are using. This feature enhances workforce mobility, facilitates remote work, and promotes efficient decision-making.
2. Mandatory Review based on Knowledge Gained on LMS Content: Integrating eDMS with Learning Management Systems (LMS) enables a streamlined process for mandatory document reviews. When employees complete training or gain specific knowledge through the LMS, the eDMS Software can automatically trigger document review assignments based on predefined criteria. This integration ensures that personnel with relevant expertise or newly acquired knowledge review and validate documents promptly, improving compliance and reducing delays.
3. Print Controls, Additional Copy Prints, Reprints: eDMS Software offer robust print controls to manage document printing within the organization. Administrators can set permissions and restrictions on printing, limiting the number of copies, and controlling who can print documents. This feature helps prevent unauthorized distribution of sensitive information, reduces unnecessary printing costs, and ensures that only approved printed copies are generated, eliminating the risk of outdated or uncontrolled prints.
4. Issuance of Documents (Controlled Copies, Drafts, Uncontrolled Copies, and Training Copies): eDMS facilitates the issuance of different document versions and types as per the defined requirements. Controlled copies, which are official and approved versions, are securely stored within the eDMS repository. Drafts allow for collaborative editing and review before final approval. Uncontrolled copies are made available for reference purposes, typically with restricted editing rights. Additionally, eDMS can manage the issuance of training copies, ensuring that the latest version of documents is available for training purposes, maintaining consistency and accuracy.
5. Reconciliation of Issuance: Reconciliation of document issuance is a critical process to ensure that the right versions of documents are distributed and accounted for. Hence, it simplifies this task by maintaining a centralized record of issued documents. It tracks the issuance status, identifies discrepancies, and provides real-time visibility into the location and status of each document. This reconciliation feature helps eliminate errors, minimize the risk of non-compliance, and ensures accurate documentation throughout the organization.
6. Version Management to Avoid Deviations on Timely Management of Versions: eDMS platforms excel at version management, ensuring that the correct document versions are used throughout the organization. By maintaining a clear version history, eDMS enables easy identification of the latest approved version and highlights any deviations or outdated versions. Notifications and alerts can be set up to inform users when a new version is available, minimizing the chances of using incorrect or obsolete documents. This feature promotes compliance, reduces errors, and fosters adherence to standardized procedures.
7. Watermarks: eDMS Software provide the option to add watermarks to documents, enhancing document security and control. This feature helps prevent unauthorized distribution, discourages unauthorized modifications, and maintains document integrity.
8. Change Control Integration and LMS System Integration: eDMS platforms often integrate seamlessly with change control systems and LMS systems. Change control integration ensures that any changes made to a document, such as revisions or updates, follow a defined change control process, allowing for proper review, approval, and documentation of changes. Integration with LMS systems ensures that employees have access to the latest versions of documents aligned with their training records, promoting consistency and compliance.
9. Anytime Reference of SOPs in Shopfloor: eDMS enables real-time access to Standard Operating Procedures (SOPs) from the shop floor or any other work area. By accessing the eDMS system through devices such as tablets or mobile devices, employees can instantly refer to relevant SOPs, work instructions, or guidelines while performing their tasks. This ensures accuracy, minimizes errors, and promotes adherence to standardized procedures, ultimately enhancing productivity and compliance.
Wrap up
In conclusion, eDMS addresses multiple challenges faced by the pharmaceutical industry. The features of the Software empower organizations to streamline processes, improve compliance, enhance document control, and facilitate efficient operations throughout the pharmaceutical sector.
However, an effective solution can streamline how the staff manages documents, resulting in a better workplace. Now that you are aware of the challenges that a powerful document management Software can help you overcome, you must find a solution to serve your business and compliance requirements.