In the ever-evolving landscape of regulated industries such as life sciences, pharmaceutical manufacturing, gene therapy and medical devices; the demand for cutting-edge technology solutions is paramount. As these sectors strive for innovation while adhering to stringent regulations as per electronic records guidelines defined in US FDA 21 CFR Part 11, EU Annexure 11 and GAMP 5 category standards; it becomes imperative to explore platforms that can seamlessly blend customization features with efficiency and provide integration among business processes.
The regulated companies have finely leveraged cloud technology to streamline business activities that address current cyber security challenges and provide customized solutions. These service offerings consist mainly of Software-as-a-Service (SaaS) and Platform-as-a-Service (PaaS). But more often than not, these companies require customized solutions for their unique needs instead of just standard solutions. Now, before delving into the same let’s first understand what are cloud solutions and their types.
Cloud solutions refer to computing services and resources delivered over the internet, commonly known as the cloud. These services include a variety of applications, storage, processing power, and more, enabling users to access and use these resources remotely rather than on their local devices or servers.
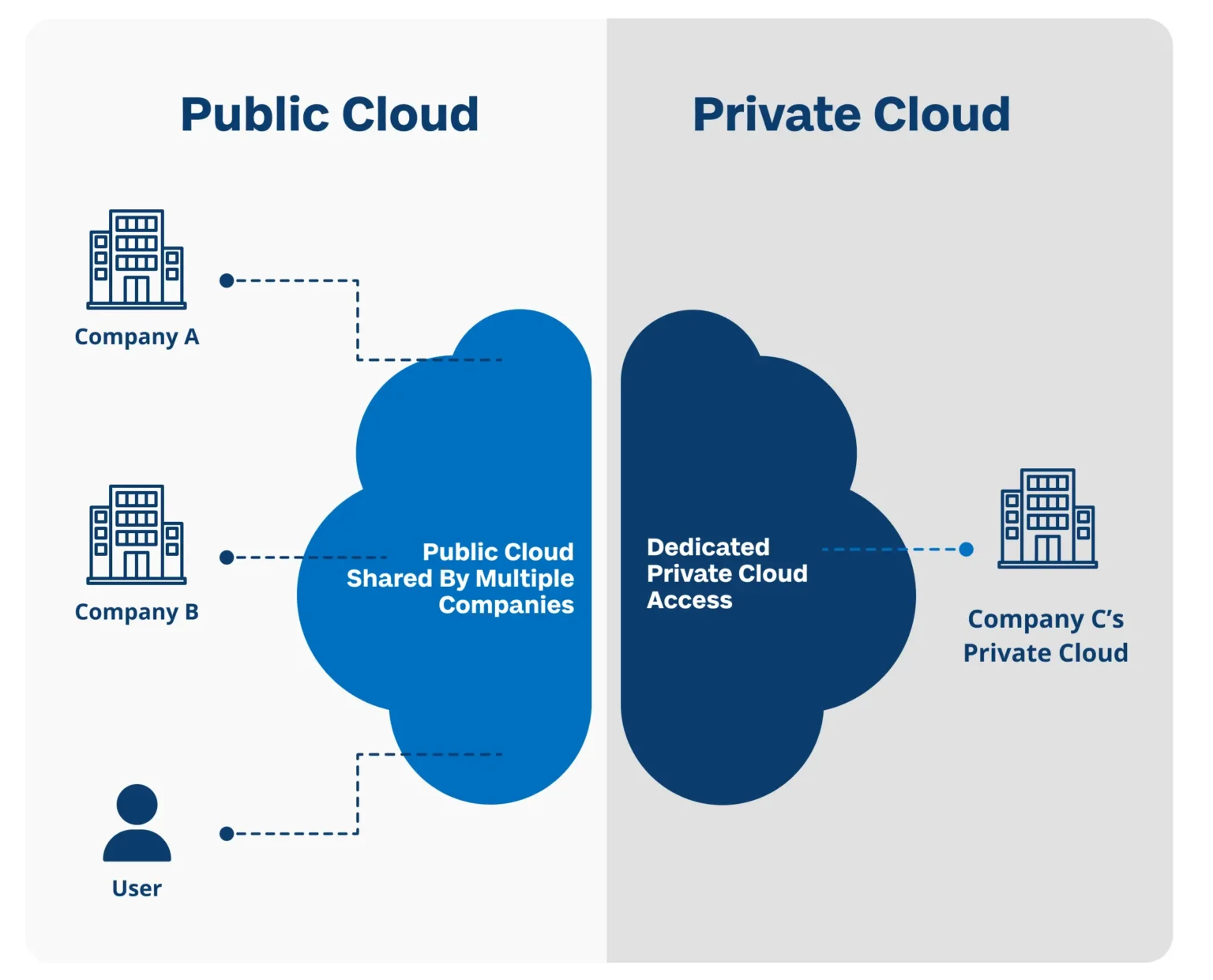
A private cloud is hosted, used and maintained by a single organization. This means it is a dedicated cloud that is solely used by an individual company who has overall control over the infrastructure and its services.
Ensures complete autonomy.
- They can customize the environment according to their own needs.
- Companies meet their compliance requirements better.
- Higher security and reduced chances of cyber attacks since data is not shared, chances of security breaches are reduced significantly.
Public cloud is a third-party managed platform that is used by multiple companies to do their regular tasks. This setting relieves companies from owning or maintaining their own infrastructure. Public cloud resources usually include virtual machines, applications and storage.
- Cost effective as companies don’t have to maintain their own infrastructure.
- Offers higher agility and scalability, can increase or decrease resources as required without impact of increasing or decreasing workload and fluctuation in business requirements.
- Promotes efficiency.
- Pay for what you use.
Types of Cloud Models
Broadly there are three types of cloud models:
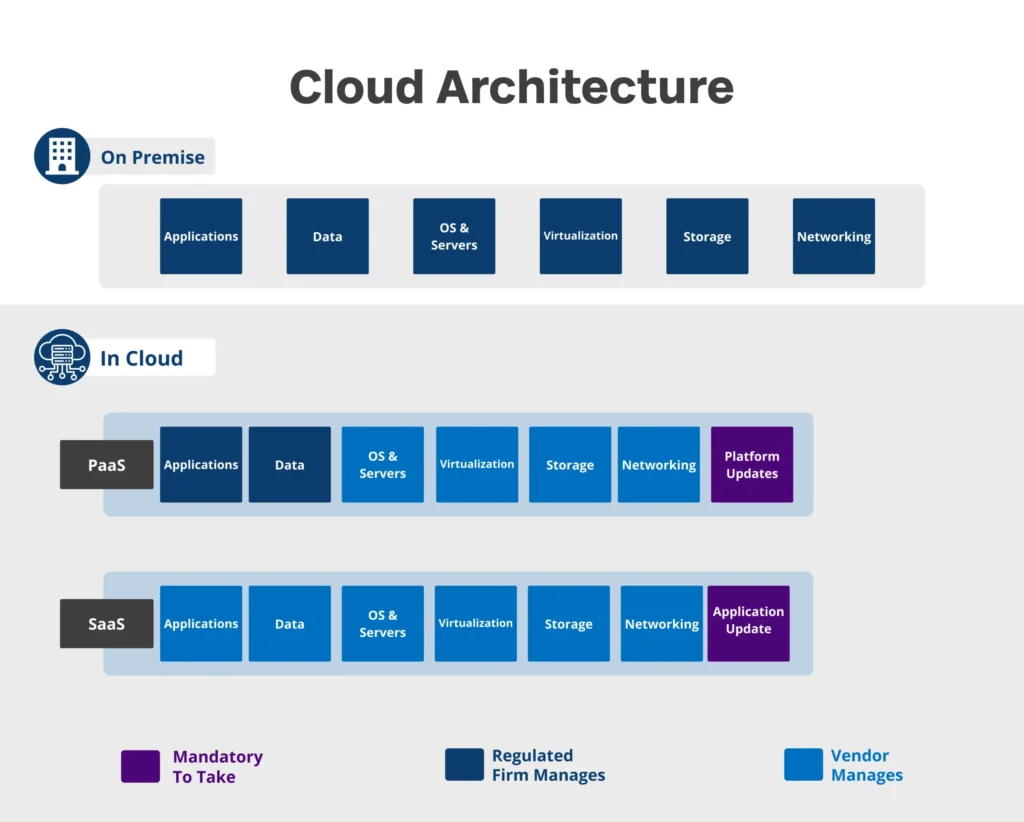
- In the IaaS (Infrastructure as a Service) model, vendors offer the entire cloud infrastructure including hardware, computing resources and data centres. This way the end user can access the hardware over the internet while paying for the service. Usually, companies sign up for virtual machines or they go for a dedicated hardware for their personal usage.
- In the PaaS (Platform as a Service) model, vendors provide users with a development and deployment environment where clients build their own applications and software. PaaS is a great solution for businesses who want personalized softwares. A low to no code environment additionally helps companies build applications with no prior knowledge of programming languages.
- Among the three Cloud models, SaaS (Software as a Service) is by far the most popular! In this model, companies offer their own software or applications to clients. It is the vendor who then takes care of the maintenance of these software. They are also responsible for the updates. Users get generic applications with SaaS.
Irrespective of SaaS’ high demand, regulated industries seek personalized solutions. As new technologies seep in every day, a sub-model of PaaS – aPaaS – outshines as a better alternative for automating highly regulated industries. Let’s look at this article to explore the benefits of adopting an Application-Platform-as-a-Service (aPaaS) solution over traditional Software-as-a-Service (SaaS) model, highlighting the advantages and proposing a novel approach – aPaaS with Commercial Off-The-Shelf (COTS) products – for enhanced adaptability, time efficiency, cost management and building of business-centric solutions without developing them from scratch.
Challenges with SaaS in Regulated Industries
1. Customization Roadblocks: Traditional SaaS solutions often come with predefined features and limited flexibility, creating roadblocks for organizations in regulated industries such as in the lifesciences, pharmaceuticals, and medical devices that have unique workflows and compliance requirements such as US FDA 21 CFR Part 11, EU Annexure 1, GAMP 4 & 5 category standards. Although in private cloud, customization is possible but comes with very high cost and maintenance.
2. Limited Control Over Updates: In regulated environments, controlling the timing and impact of software updates is crucial. SaaS solutions may force updates that can disrupt workflows. To ensure processes run smoothly and as expected companies need to validate the updates which is pretty time consuming and shoots up costs.
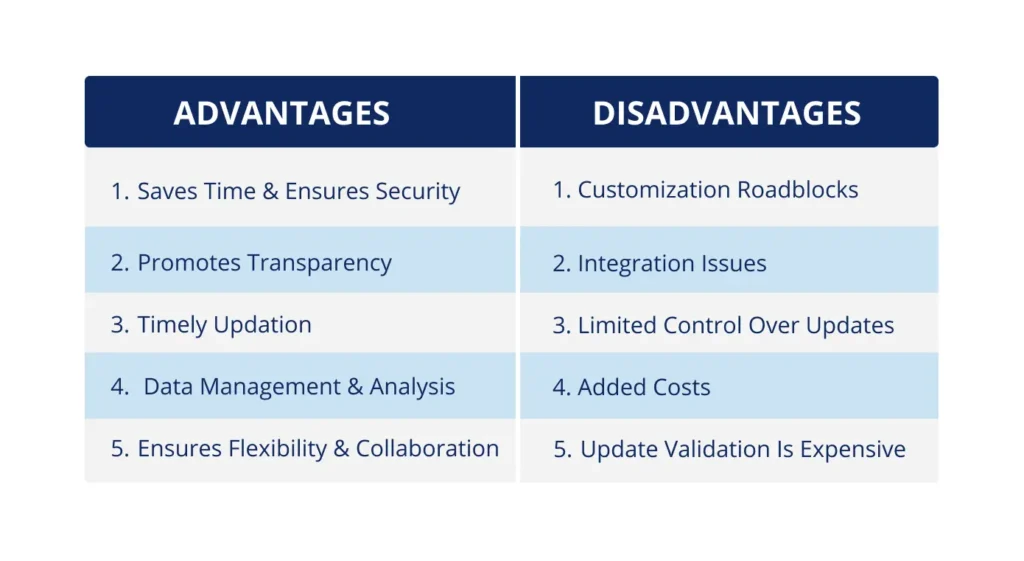
3. Integration Issues: No service provider typically offers a comprehensive suite of SaaS products that fully meet a business’s diverse needs. This leads organizations to depend on multiple SaaS providers, resulting in the management of multiple accounts and an associated high cost of integration.
4. Added Costs: Services from multiple vendors for different products also involve additional costs. Additionally, standard Service Level Agreements (SLAs) offered by SaaS providers may not be sufficient for regulated environments, requiring negotiations for enhanced uptime, security, and data recovery guarantees, which would be an added cost to the organisations. This is a key issue as every company runs on a specific budget.
The Essence of aPaaS in Regulated Industries
1. aPaaS as a Subset of PaaS
Recognizing aPaaS as a subset of Platform as a Service (PaaS) sheds light on its specialized role in providing tailored solutions for application development. aPaaS providers leverage Low Code No Code (LCNC) methodologies, making application development more accessible and efficient.
2. LCNC Methodology
The LCNC methodology empowers businesses to rapidly develop applications, reducing time-to-market. By offering Low Code platforms on their cloud infrastructure, service providers facilitate the creation of customized applications without the need for extensive coding expertise.
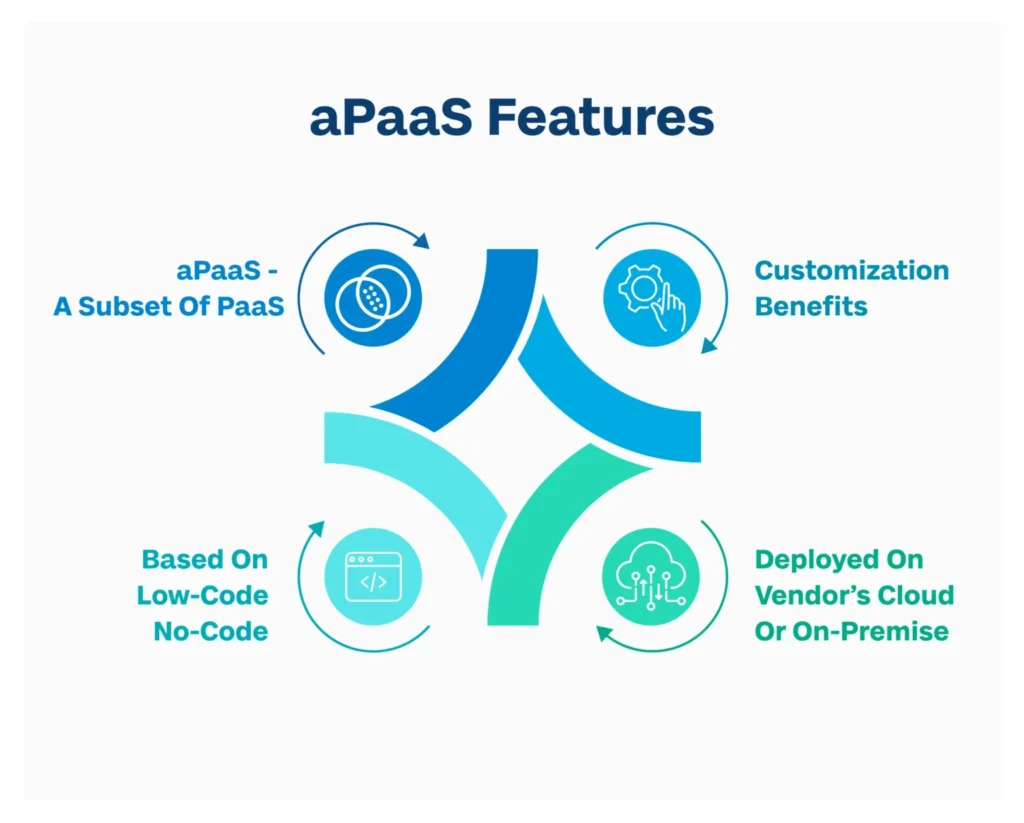
3. Customization Benefits
One of the key advantages of developing products on such Low Code platforms is the flexibility they offer to accommodate customer-specific requirements. This adaptability is particularly beneficial when organizations seek tailored solutions to meet their unique business needs, enabling a more agile response to changing market dynamics. Users get this advantage when they opt for an aPaaS platform.
4. Deployment Methods
There are two ways of deploying an aPaaS platform:
- On Cloud: Usually, aPaaS vendors deliver Low Code platforms on their own cloud infrastructure. In this case the vendor is responsible for maintaining the platform and infrastructure. Users can leverage the platform to build applications of their own choice.
- On-Premise: For organizations with existing data centers and virtual servers, hosting Low Code platforms (aPaaS) on-premise is a viable option. In this scenario, businesses assume responsibility for managing the infrastructure while the service provider offers platform support. Therefore, businesses are more accountable for the application created and their performance is heavily dependent on the on-premise infrastructure.
Advantages of aPaaS in Regulated Industries:
1. Tailored Solutions: aPaaS empowers organizations to build custom applications tailored to their unique needs. This is particularly advantageous in regulated industries such as lifesciences, pharmaceuticals, and medical devices where workflows can be complex and must align with strict guidelines.
2. Reduced Compliance Burden: aPaaS solutions for life sciences, pharmaceutical manufacturing, gene therapy and medical devices must comply with regulations such as US FDA 21 CFR Part 11, EU Annexure 11, GAMP 5 category standards, HIPAA, SOC 2 etc. Many aPaaS platforms include pre-built compliance features and frameworks. This allows organizations in regulated industries to quickly and easily ensure their applications meet compliance requirements. This can significantly reduce the burden of compliance for regulated organizations.
3. Greater Control Over Data: With aPaaS, organizations retain greater control over their data, facilitating compliance with industry regulations.
4. Efficient Integration with Existing Systems: aPaaS solutions allow seamless integration with existing systems, fostering interoperability, and reducing disruptions during the adoption phase. This is crucial for industries where legacy systems are prevalent. Also, multiple applications are integrated on a single platform that work by sharing data. This helps in seamless business proceedings.
5. Reduced Cost: With aPaaS, development teams can leverage pre-built modules, components, and templates. This accelerates the application development process, saving time and reducing the overall development cost.
6. Time Efficiency: Ready-made functionalities provided by aPaaS platforms eliminate the need for coding from scratch. Developers can focus on customizing these components, significantly reducing the time required to bring an application to market.
COTS in the Mix
Understanding COTS: Commercial Off-The-Shelf (COTS) products are ready-to-use applications that vendors usually offer on top of a low code platform (aPaaS). COTS on Lowcode helps subside all disadvantages where only the benefits can be reaped.
Deployment Method: COTS products or applications are deployed on a customer’s infrastructure. When hosted on service providers’ clouds, they fall under the category of Software as a Service (SaaS).
Customization Benefits: COTS products (applications) offered by vendors on Low Code platforms provide customers the flexibility to accommodate customer-specific requirements. This synergy allows for tailored solutions that align with the unique needs of regulated industries.
Some examples of COTS are eQMS, DMS, LMS, LIMS, RIMS etc.
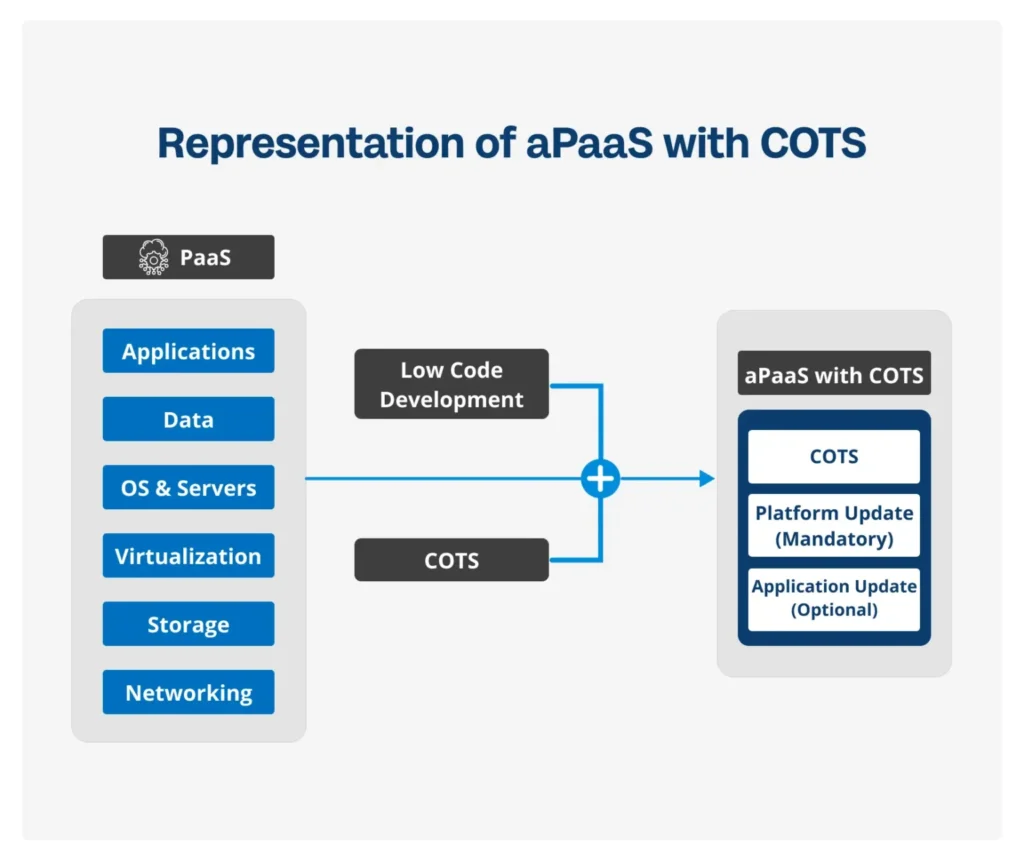
Proposing aPaaS with COTS
1. Standard Products with Customization Benefits: aPaaS with COTS products (applications) offers the dual advantage of getting ready-made solutions with the flexibility of customized development. Organizations can use COTS for standard processes and easily customize with aPaaS when needed. aPaaS allows them to develop custom add-ons or extensions that add functionality to existing COTS solutions.
2. Cost-Effective Scalability: Leveraging COTS for standard functionalities reduces development costs, while aPaaS facilitates cost-effective customization. This hybrid model ensures scalability without compromising budget constraints.
3. Streamlined Compliance: COTS products often come with built-in compliance features. Integrating them with aPaaS allows organizations to extend these features to custom applications, ensuring a streamlined approach to compliance and regulatory adherence.
4. Rapid Development and Deployment: The combination of aPaaS with COTS enables rapid development and deployment of applications. This agility is crucial in regulated industries where timely responses to changing requirements are essential.
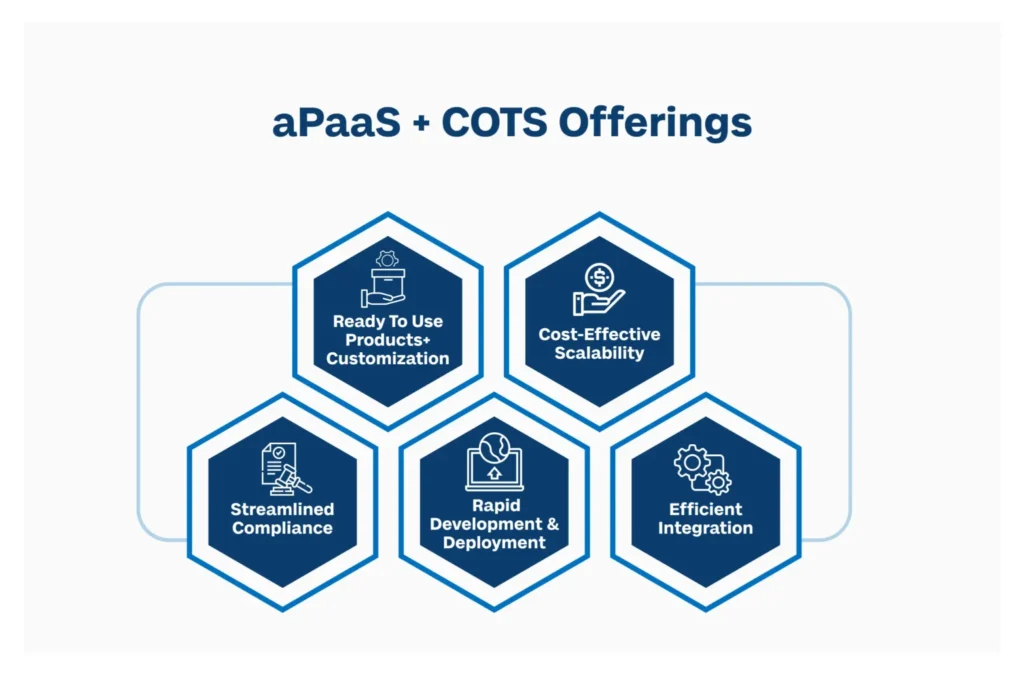
5. Efficient Integration: aPaaS providers can consolidate various services under a single platform streamlining integration, reducing complexity and overall costs. Applications in this case is integrated using API (Application Programming Interfaces) that helps in exchange of data and performance of key tasks. For example, a Quality Management System (QMS) can easily ensure if a person is trained to do a work by its integration with a Learning Management System (LMS) hosted in aPaaS.
As regulated industries continue to navigate the delicate balance between innovation and compliance, the adoption of aPaaS with COTS emerges as a strategic move. This approach not only addresses the limitations of traditional SaaS models but also provides a tailored, cost-effective, and scalable solution. By embracing aPaaS with COTS, regulated industries such as life sciences, pharmaceutical manufacturing, gene therapy and medical devices propel themselves into a realm where technology serves as an enabler, not a restriction. So, are you ready to unlock efficiency and embrace a future where compliance and innovation coexist harmoniously? The time is now. Achieve business process excellence using aPaaS with COTS solutions.