Quality audits specifically examine how well product designs and processes meet specified standards and regulatory compliances. They are performed periodically by internal people within the organization or externally by a quality auditor or an audit team.
Quality Audit is an essential part of product processing, testing, and the product distribution system, especially in regulated industries where manufactured goods come in direct contact with the users. Industries such as Lifesciences, Medical Devices, Food and beverages, Beauty and Cosmetics, Gene Therapy, etc, fall under this category. Other industries have their own set of quality audits, but these sectors need specialized attention. Quality audits in these fields are, hence, known to be rigorous.
Quality Audits Definition
Quality audits are independent, systematic evaluations of processes, systems, and products within an organization. It helps in determining business compliance with standardised procedures, current regulations and best practices.
During quality audits, if any gap is identified, the situation is assessed to understand the impact on the product. Also, investigation is carried out to find the root cause of the gap. Accordingly, appropriate corrective and preventive action is implemented to eradicate similar issues in the future.
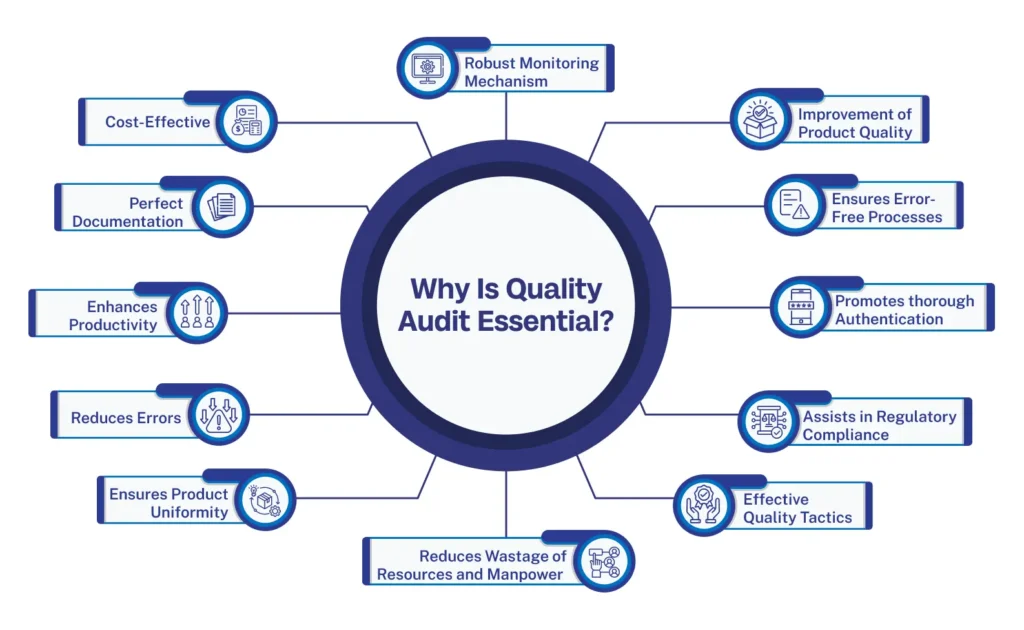
Why is Quality Audit Essential?
Quality audits help companies access their performance and record outcomes in documents. This is the best way businesses can optimize their growth and generate more revenue. Companies can benefit in the following ways from Quality audits:
- Robust Monitoring Mechanism
- Improvement of Product Quality
- Ensures Error-free Processes
- Promotes thorough Authentication
- Assists in Regulatory Compliance
- Effective Quality Tactics
- Enhances Productivity
- Ensures Product Uniformity
- Reduces Errors
- Reduces Wastage of Resources and Manpower
- Perfect Documentation
- Cost-Effective
Types Of Audits In Quality Management
Internal Quality System Audit: Evaluation of systems and processes within an organization is called Internal Quality System Audit. It ensures effectiveness, compliance, and improvement of internal quality management processes. Carried out by internal professionals and quality assurance teams, internal quality system audit helps companies identify areas for improvement, thus fostering operational excellence.
Supplier Audit: Businesses need to evaluate external suppliers based on criteria such as time, product quality, cost-effectiveness, and certification to ensure they meet the quality standards of the organization. Real-time transparency obtained from supplier audits helps businesses and suppliers jointly monitor purchase order activities and address issues of errors and non-conformities.
Production Team Audit: Audits on the activities performed by the production team is called Production Team Audit. Businesses audit their actions for operator acceptance or certified operator programs. This is done to requalify their skills. Auditors review training records, change in processes and past activities to ensure that everything meets quality standards.
Safety Audit: This is one of the essential audits operational in organizations. Safety audits help companies keep their employees safe by reviewing equipment and evaluating the safety steps at place. Companies need to have structured policies to prevent accidents or injuries. This enables safer environment for all employees.
Facilities Audit: The audit keeps a check on the quality of an organization’s assets such as buildings and equipments. Auditors need to monitor ACs, HVAC (heating, ventilation, and air conditioning), water tankers, manufacturing equipment, technology, etc. Such audit ensures safety and improves quality outcomes.
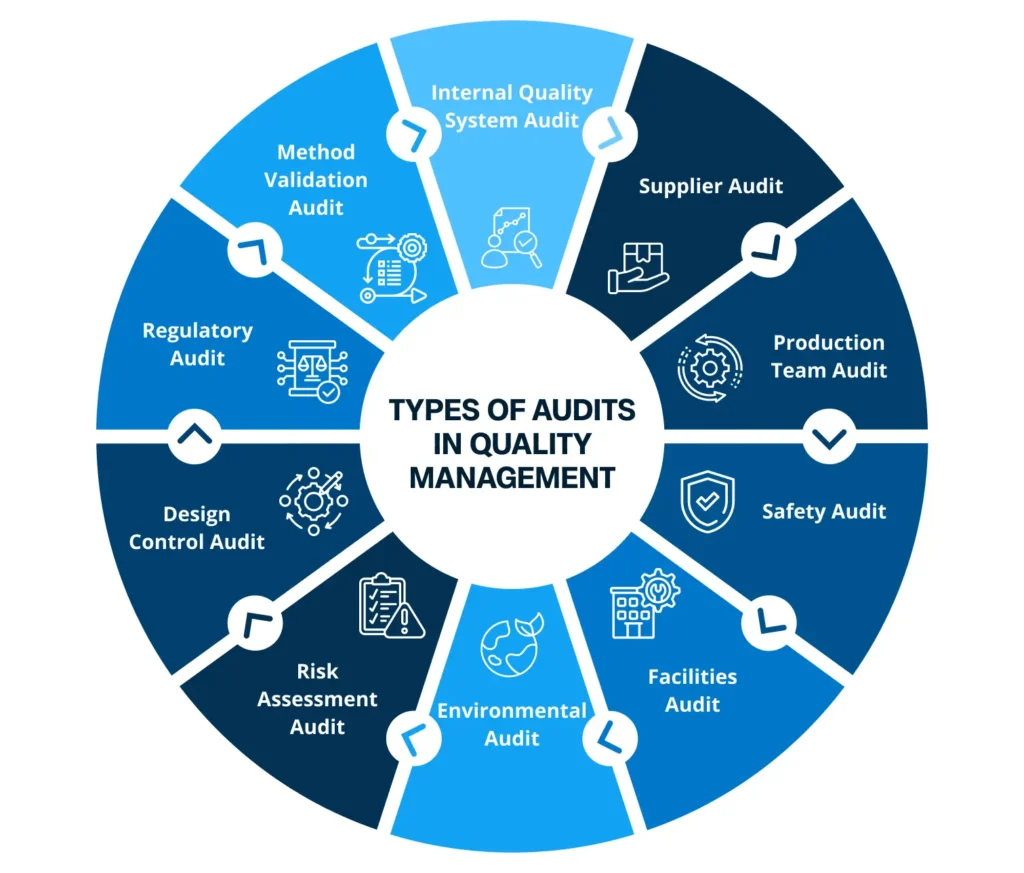
Environmental Audit: Environmental audits ensure that workplaces are free from pollution and toxins. It helps identify potential risks and build plans to meet safety standards like cGMP, OSHA, etc. The audit team additionally keeps check on employees for following the standards and usage of proper disposal techniques.
Risk Assessment Audit: The risk assessment audit especially helps in identifying workplace risks. Each risk is put under certain categories and aligned measures are specified to prevent quality issues, accidents, machine failures, natural disasters, etc.
Design Control Audit: It ensures businesses follow proper, compliant, and systematic methods to produce safe and high-quality products. The audit team designs plans, provides inputs and outputs to observe that specific criteria are met, and potential risks are analyzed before occurrence.
Regulatory Audit: This audit ensures that organizations follow specific guidelines laid down by regulatory agencies. Auditors not only review quality practices but also gather important data to check grey areas where rules are not being followed.
Method Validation Audit: Regulatory bodies such as FDA put forward method validation audit to check if testing methods in manufacturing are standardized, consistent and well documented. It emphasizes the reliability and accuracy of products made by humans.
Why Quality Management Software?
Managing audits manually can be tedious and prone to errors. QMS software in such a scenario, can really be the best solution! Quality Management Software takes care of quality audits and keeps track of all business operations. The solution comes with seamless data integration and guaranteed authentication that ensures transparency and accuracy. Businesses have been leveraging on Quality Management Softwares (QMS) to guarantee process and product quality and, compliance with regulations.
AmpleLogic’s eQMS software is specially crafted for highly regulated sectors such as Lifesciences, Medical Devices, Food & Beverages, Beauty and Cosmetics, Gene Therapy and so on.
AmpleLogic’s eQMS software renders 11 specific modules: CAPA, Change Control, Market Complaints, Deviations, Audits, Vendor Qualification, Out of Specification, Out of Trend, Lab Incident Reporting. All these modules are seamlessly integrated with one another.
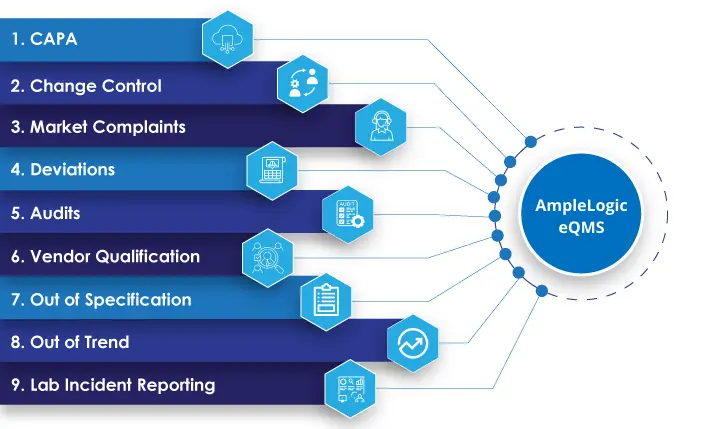
AmpleLogic eQMS System
AmpleLogic also has other COTS products like Document Management System (DMS), Learning Management System (LMS), Regulatory Information Management System (RIMS), Electronic Batch Management System (eBMR), Environmental Monitoring Software (EMS), etc that are integrated with eQMS to offer best quality management!
How is AmpleLogic’s eQMS the Best Solution?
- Round-the-Clock Accessibility
- Peaked for Perfection
- Quick Alerts and Prompts
- Guarantees Reviewed and Verified Processes
- Keeps Track Records
- Built-in Emailing Feature
- Appealing Visual Representation
- No Geographical Hindrances
- Enhanced Transparency
- Compliant to International Regulations such as US FDA 21 CFR Part 11, EU Annexure 11, GAMP 5 standard, GMP, Alcoa+ Principles, etc
To gain a more in-depth understanding of our eQMS product, request a demonstration at: Click here to Request Demo