AmpleLogic Quality Suite
QUALITY SUITE
AmpleLogic Quality Suite
AmpleLogic Quality Suite encompasses three essential products
AmpleLogic Quality Suite encompasses three essential products i.e., Document Management System, Learning Management System and Quality Management System. All these products seamlessly integrate with one another and with external systems. With our GAMP solution suite, the pharma industry can now streamline operations and comply with quality and regulatory standards.
Our three-pack solution is ideal for regulated industries owing to its regular updates and in-built validation processes. As regulated industries need technological intervention to optimize processes with accuracy, AmpleLogic’s Quality Suite proves unbeatable! The three-pack solution complies with US FDA 21 CFR Part 11, EU Annexure 11, GAMP 5 standard, GMP, Alcoa+ Principles, etc. Try AmpleLogic’s comprehensive software suite to ace business process excellence today!
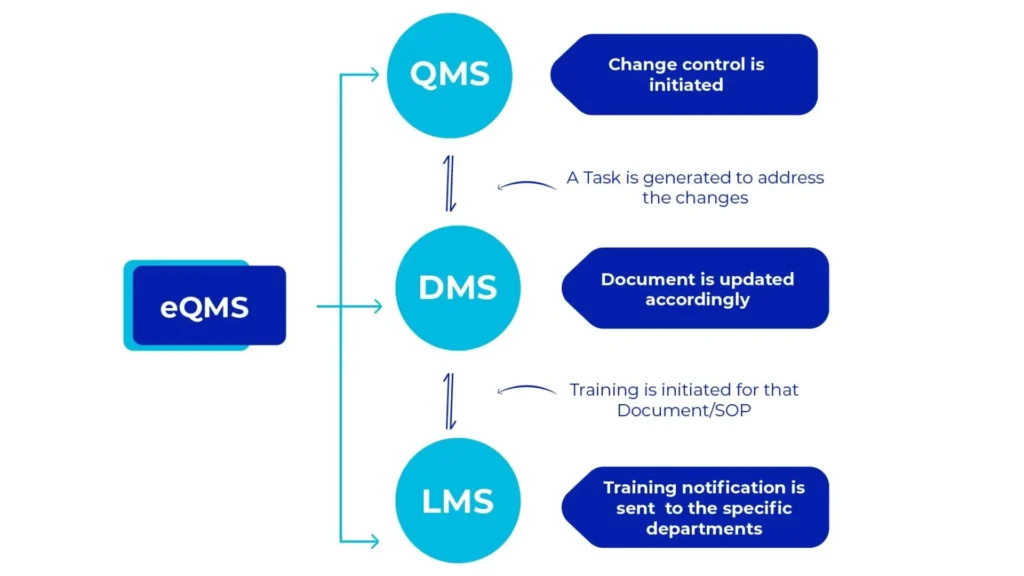
AmpleLogic Quality Suite Modules
CAPA Management Software
Efficiently manage corrective and preventive action processes from initiation to evaluation, ensuring compliance and enhanced quality control with AmpleLogic's CAPA Management module.
Change Control Management System
Streamline automation, ensure compliance, and boost productivity throughout the change management lifecycle with AmpleLogic Change Control software, tailored for GMP-driven industries.
Document Management
Efficiently manage document lifecycle with our integrated Document Management System (DMS). Ensure compliance and streamline processes with version control, document tracking, and secure access controls.
Training Management
Empower your team with our robust Learning Management System (LMS) designed to streamline learning and development processes. Track employee training progress, certifications, and compliance requirements effortlessly.
Market Complaints Management
Streamline complaint intake, investigation, and resolution processes with AmpleLogic's comprehensive software, ensuring compliance and enhancing customer satisfaction in regulated industries.
Deviation Management
Automate and streamline deviation management in the life sciences industry with AmpleLogic Deviation Manager. Ensure compliance with FDA 21 CFR part 11 and EU Annex 11 while enhancing operational efficiency and reducing recurrence.
Audit Management Software
Enhance audit processes for pharmaceutical, biotech, and medical device industries with AmpleLogic's Audit Management Software. Automate planning, execution, reporting, and follow-up to meet GMP audit and compliance requirements.
Vendor Qualification Management
Streamline vendor assessment processes, automate workflows, and ensure compliance with AmpleLogic's Vendor Qualification Management software, enhancing efficiency and quality in pharmaceutical operations.
Out of Specification (OOS)
Ensure compliant management of out-of-specification results with AmpleLogic's OOS Software. Streamline lab investigations, automate reporting, and improve cross-functional collaboration to enhance workflow efficiency.
Out of Trend (OOT)
Manage out-of-trend results in quality control labs efficiently with AmpleLogic's OOT Software. Automate reporting, streamline investigation workflows, and ensure regulatory compliance for swift resolution of quality issues.
Incident Reporting Software
Manage incidents comprehensively with AmpleLogic Lab Incident Reporting (LIR) software. Facilitate efficient reporting, assessment, and resolution of quality events while ensuring regulatory compliance.
Product Recall Management
Efficiently handle product recalls with AmpleLogic's Product Recall Management software. Swiftly identify, assess, and execute recalls while ensuring regulatory compliance and protecting brand reputation.
Quality Risk Management (QRM)
Proactively identify, assess, and mitigate risks with AmpleLogic's QRM solution. Utilize advanced analytics and streamlined workflows to support risk-based decision-making and enhance overall quality performance in regulated industries.
Features of AmpleLogic Quality Suite
AI-Enabled Learning
AI enables quick generation of question banks for evaluation purposes. This enables streamlined learning and assessments with lesser manual labour and more efficiency
Quality Assurance
Streamline operation, and ensure quality and regulatory compliance with advanced CAPA, change control and deviation handling, document management, etc.
Document Comparison
Easily compare changes made between different versions of a document
Streamline Operations
Optimize tedious operations using AmpleLogic’s Quality Suite to ensure accuracy, efficiency, and quality
MS Word Integration
Streamline document creation, editing and management within familiar Word environment
Real-Time Monitoring
Real-time monitoring of data with automated alerts and notifications during escalations and deviations
Training Control
Tailor and control training programs for employees based on their department, designation, and specific job roles to ensure impactful learning experience
Audit & Quality Management
Schedule and track audits, also handle deviations, OOT, OOS, etc using the AmpleLogic QMS system
Documentation Efficiency
Streamline documentation lifecycle of SOPs, STPs, BMRs, reports, etc. with superior system integration, data security, version control, saved documentation templates, etc.
Version Control
Easily update SOPs, STPs, etc. and keep track of all changes in documents to eliminate confusion
Continuous Improvement
AmpleLogic’s Quality Suite enables continuous improvement of processes through risk assessment and mitigation and real-time monitoring
Workflow Configuration
Workflow can be configured to each business's needs, according to their processes and operations
Role-based Access
Access is provided based on role and responsibilities. This includes training and other criteria that might be required for a process
Cross-Functional Department Collaboration
Helps departments collaborate and enhances communication for streamlining processes
Role-Based Training
Personalised training for each employee as per requirement of role and responsibility.
Enhanced Compliance Training
Learn about regulations and SOPs that are ever-evolving in the dynamic realm of life sciences
Bio-Metrics System Integration
Use biometric fingerprint technology to track attendance accurately and securely, allowing tailored training schedules for present employees
Data Analysis and Security
Get advanced data analysis and security with robust encryption, access controls and real-time monitoring features
Seamless Integration
Integrate seamlessly with internal and external systems to collect data, analyze them and provide real-time insights
Single Sign-in
Single sign-on across all systems saves time and effort. It streamlines major processes with faster sign-in and control
Saves Time
Saves a lot of time owing to streamlined processes, single-user sign-in, and lesser manual labour
Regulatory Compliance
Comply with global regulations like US FDA 21 CFR Part 11, EU Annexure 11, GAMP 5 standard, GMP, Alcoa+ Principles, etc
Hear From Our Customers
Frequently Asked Questions
What is the primary purpose of AmpleLogic APQR?
AmpleLogic APQR is designed to streamline the Annual Product Quality Review process, ensuring compliance, and providing detailed insights into product quality trends.
How does the software handle deviations?
The software triggers immediate alerts for deviations, allowing timely corrective actions to maintain product quality.
Can AmpleLogic APQR integrate with existing systems?
Yes, AmpleLogic APQR seamlessly integrates with various systems, including LMS, QMS, BMS, and MES/eBMR for comprehensive data capture.
What statistical analysis does the software perform?
The software performs statistical analysis on parameters such as Assay, Water Content, PH, Specific Impurities, and Total Impurities providing valuable insights.
Is AmpleLogic APQR compliant with industry standards?
Yes, AmpleLogic APQR complies with major standards including 21 CFR PART 11, MHRA, EU Annex 11, etc ensuring regulatory adherence.
Contact us
Your Pharma Automation Starts Here
We’re here to address your inquiries and assist you in identifying the solutions that best align with your requirements. Here’s why choosing us is your strategic advantage:
Your benefits:
- Client-oriented
- Independent
- Competent
- Results-driven
- Problem-solving
- Transparent
What happens next?
1
Schedule a call at your convenience
2
Discovery and consultation session
3
Get your custom proposal


























