AI Deviation Management software
Predict, Prevent, and Resolve Deviations with AI-Driven Insights
AmpleLogic AI-Powered Deviation Management Software
AmpleLogic AI Deviation Management Software revolutionizes quality control in the pharmaceutical and life sciences industries by leveraging AI-driven automation to streamline deviation handling. This advanced solution ensures real-time identification, classification, and resolution of deviations, reducing manual effort while maintaining compliance with FDA, EU GMP, and MHRA regulations. Seamlessly integrating with existing Quality Management Systems (QMS), it eliminates data silos, enhances operational efficiency, and enables swift deviation detection and mitigation. With robust root cause analysis (RCA) capabilities, the software identifies underlying issues, prevents recurrence, and supports proactive compliance. Automated workflows simplify documentation, deviation tracking, and corrective actions, ensuring regulatory adherence while minimizing risks. AI-powered alerts accelerate issue resolution, reducing downtime and improving productivity.
AmpleLogic AI also delivers predictive insights, smarter recommendations, and accurate impact analysis, transforming deviation management into a seamless, intelligence-driven process. By combining automation, analytics, and compliance assurance, this software empowers organizations to enhance decision-making, mitigate risks, and drive operational excellence.
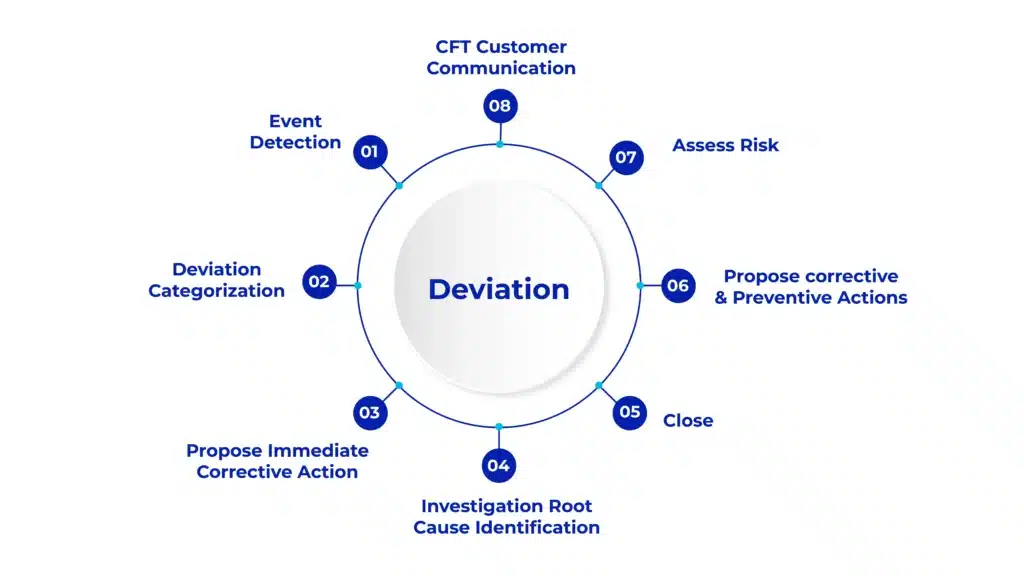
Key Features
Automated Deviation Management
Handles both intended and unexpected deviations from reporting to closure.
Quality Metrics and Reporting
Allows thorough examination of activities associated with each process, facilitating CAPA implementation.
Flexible Workflow
Customizable workflows with automated email alerts for verification and escalation.
Audit Trail and Compliance
Provides a reliable audit trail compliant with regulations such as 21 CFR part 11 and EU Annex 11, with support for e-signatures and data integrity.
Root Cause Analysis (RCA)
Facilitates RCA processes with various assessment methods such as fishbone diagrams and 5 why's analysis.
Integration and Connectivity
Integrates with other quality processes and systems like CAPA, Change Control, and Document Management System.
Remote Accessibility
Accessible 24/7 from anywhere within the company.
Data-Driven Decision Making
Compiles intelligent data to reduce deviation recurrence and improve operational efficiency over time.
Automation and Live Tracking
Automates alerts, notifications, and live tracking of deviations, reducing manual entries.
Tailored Workflows and Reporting
Customizable workflows and reports to fit the organization's needs.
Graphical Representation
Metrics, reports, and trends presented graphically for comprehensive understanding.
Compliance with Regulatory Standards
Meets electronic record standards defined by various regulatory bodies.
Benefits of AmpleLogic's Deviation Management Software
AI-Driven Recommendations
AI-generated recommendations for deviation management
Enhanced Impact Analysis
Identifies affected departments for swift collaboration and resolution
Predictive Time Estimates
Predicts resolution time for better planning and efficiency
Improved Compliance
Meets regulatory standards such as FDA, 21 CFR part 11, and EU Annex 11

Frequently Asked Questions
What is AmpleLogic Deviation Management Software?
AmpleLogic Deviation Management Software automates deviation reporting and resolution processes for streamlined compliance in the life sciences industry.
How does it simplify workflow management?
AmpleLogic offers customizable workflows with automated alerts and notifications for efficient deviation management.
Can it facilitate data-driven decision-making?
Yes, AmpleLogic provides comprehensive reporting and graphical representation for informed decision-making based on deviation data.
What stages are involved in the AmpleLogic Deviation Management process?
The process includes initiation and reporting, investigation and root cause analysis, CAPA implementation and tracking, and closure and documentation.
How can I get started with AmpleLogic Deviation Management Software?
Schedule a consultation today to learn more about implementing AmpleLogic for your organization’s deviation management needs.
Contact us
Your Pharma Automation Starts Here
We’re here to address your inquiries and assist you in identifying the solutions that best align with your requirements. Here’s why choosing us is your strategic advantage:
Your benefits:
- Client-oriented
- Independent
- Competent
- Results-driven
- Problem-solving
- Transparent
What happens next?
1
Schedule a call at your convenience
2
Discovery and consultation session
3
Get your custom proposal

























