Stability Testing Software
Empower Regulated Industries with
AmpleLogic Stability Testing System
Streamlined, AI-Enabled Stability Testing with AmpleLogic
AmpleLogic Stability Testing Software is a comprehensive, compliance-driven solution built to digitally manage the complete lifecycle of pharmaceutical stability studies within regulated environments. The platform enables structured, protocol-driven study design that standardizes stability programs across long-term, accelerated, and ongoing studies, ensuring consistency and alignment with global regulatory expectations. Automated scheduling and pull-point management reduce manual coordination while ensuring timely execution of stability activities, supported by end-to-end sample incubation tracking that provides full visibility into sample location, chamber allocation, and study status at every stage.
Streamlined, AI-Enabled Stability Testing with AmpleLogic
AmpleLogic Stability Testing Software is a comprehensive, compliance-driven solution built to digitally manage the complete lifecycle of pharmaceutical stability studies within regulated environments. The platform enables structured, protocol-driven study design that standardizes stability programs across long-term, accelerated, and ongoing studies, ensuring consistency and alignment with global regulatory expectations. Automated scheduling and pull-point management reduce manual coordination while ensuring timely execution of stability activities, supported by end-to-end sample incubation tracking that provides full visibility into sample location, chamber allocation, and study status at every stage.
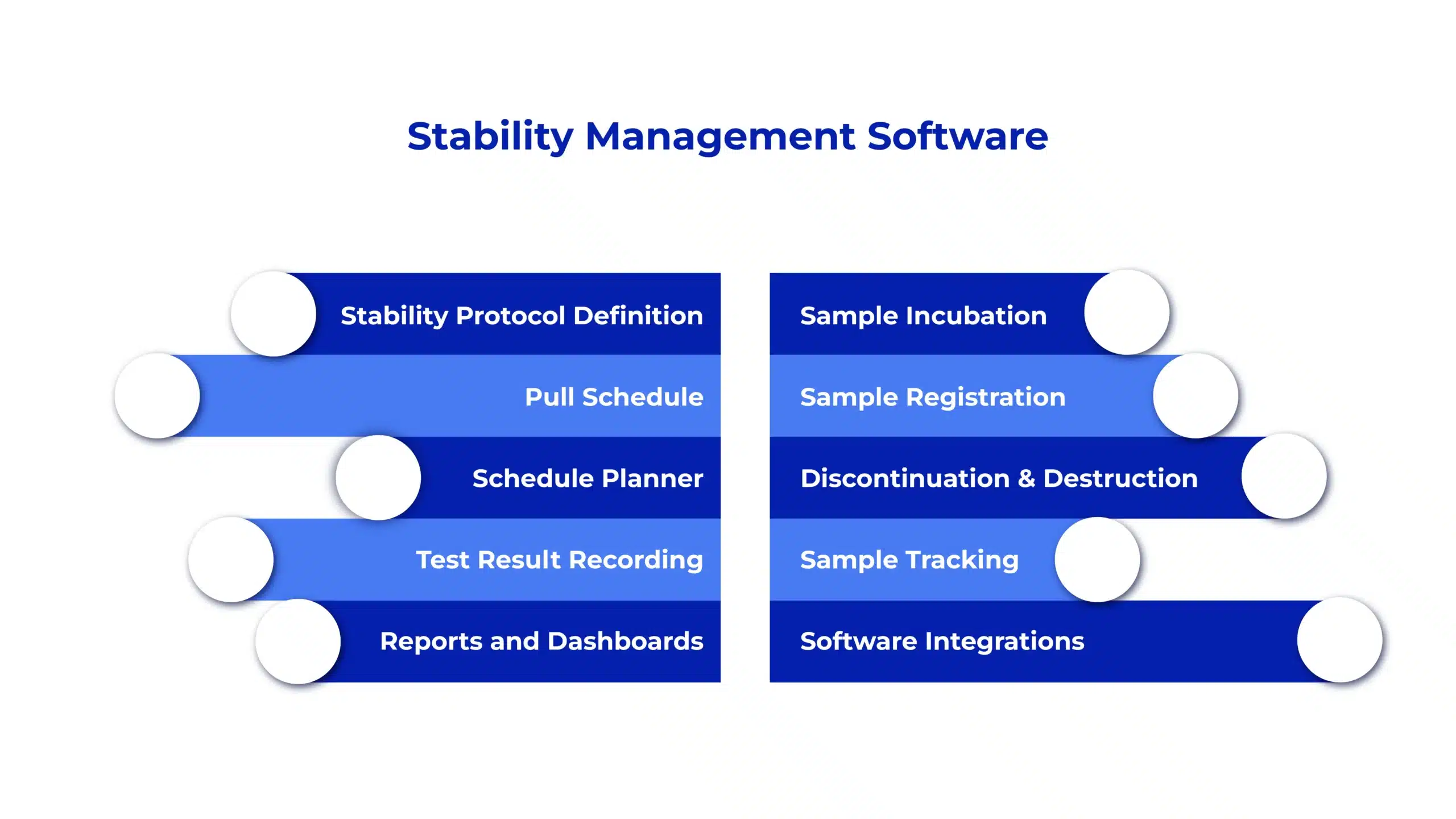
AmpleLogic Stability Software Features
This is the heading
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
Protocol Definition
Define and customize stability protocols based on product, batch, storage conditions, test intervals, and study duration to ensure standardized and compliant study execution.
Sample Incubation & Tracking
Track stability samples throughout their lifecycle with clear visibility into incubation status, chamber allocation, tray placement, and sample location.
Automated Scheduling & Calendar
Automate pull-point scheduling with calendar-based views, reminders, and alerts to ensure timely execution of stability activities and avoid missed intervals.
Test Result Recording & Controlled Analysis
Capture stability test results in a structured and secure manner. AI-assisted data validation helps identify inconsistencies or missing entries, supporting reliable trend visibility across defined intervals.
Discontinuation Management
Manage planned or unplanned discontinuation of stability studies with proper documentation, traceability, and controlled workflows to maintain compliance.
Comprehensive Reporting
Generate stability study reports along with parameter-wise trend analysis across intervals. AI-supported data summarization helps surface meaningful patterns and supports faster review.
Customizable Dashboards
Access role-based dashboards that highlight critical studies, upcoming pull points, chamber utilization, alerts, and key reports for quicker decision-making.
Chamber & Tray Availability Status
Monitor chamber occupancy and tray availability in real time to efficiently plan stability studies and optimize resource utilization.
System Integrations
Integrate seamlessly with existing laboratory and quality systems to ensure smooth data flow, reduced duplication, and end-to-end process continuity.
Error Reduction & Data Integrity
Reduce manual errors and data integrity risks through automated workflows, AI-assisted checks, audit trails, electronic signatures, and controlled access.
Unlock the Benefits of AmpleLogic Stability Software
Ensure complete and accurate traceability of stability samples, studies, and results throughout their lifecycle.
Streamline stability testing processes using automation and AI-enabled assistance to reduce manual intervention and improve productivity.
Support GMP-aligned stability management with controlled processes, documented workflows, audit trails, and regulatory-ready reporting.
Minimize data integrity risks through standardized processes, automation, and AI-assisted validation that helps flag potential issues early.
Leverage structured reports, trend visibility, and AI-supported insights to enable timely, data-driven decisions across stability programs.

Elite Pharma optimizes stability management with AmpleLogic for accurate results and robust processes.
Frequently Asked Questions
Can AmpleLogic Stability Software handle various stability study protocols?
Yes, our software allows for customization of stability protocols to meet your specific needs.
How does AmpleLogic ensure data integrity in stability testing?
Our software automates processes, minimizing the risk of data integrity errors inherent in manual methods.
Can AmpleLogic Stability Software integrate with other systems?
Absolutely, our software seamlessly integrates with ERP, LIMS, and other systems to enhance workflow efficiency.
Does AmpleLogic Stability Software offer comprehensive reporting capabilities?
Yes, our software provides detailed reports for thorough analysis and decision-making.
How can I schedule a consultation to learn more about AmpleLogic Stability Software?
Simply click on the “Schedule a Consultation Now” button or contact us directly to book a consultation with our experts.
Contact us
Your Pharma Automation Starts Here
We’re here to address your inquiries and assist you in identifying the solutions that best align with your requirements. Here’s why choosing us is your strategic advantage:
Your benefits:
- Client-oriented
- Independent
- Competent
- Results-driven
- Problem-solving
- Transparent
What happens next?
1
Schedule a call at your convenience
2
Discovery and consultation session
3
Get your custom proposal

















