
In pharmaceutical manufacturing, precision and quality are paramount. Even minor deviations in critical process parameters (CPP) or critical quality attributes (CQA) can lead to significant compliance risks and costly recalls. Continued Process Verification (CPV) is a proactive approach that ensures consistent process performance and product quality through real-time data monitoring and analysis.
With evolving regulatory expectations from agencies like the FDA, EMA, and ICH, CPV is no longer just a best practice – it is a necessity. Modern CPV platforms, such as AmpleLogic’s CPV in APQR dashboard, are revolutionizing manufacturing by integrating data from various sources, validating process efficiency, and providing actionable insights.
In an industry where a 0.1% deviation can trigger million-dollar recalls, platforms like AmpleLogic’s CPV dashboard are rewriting the rules of quality assurance. Let’s explore how these systems are transforming raw data into lifesaving precision.
The Challenges in CPV in Manufacturing
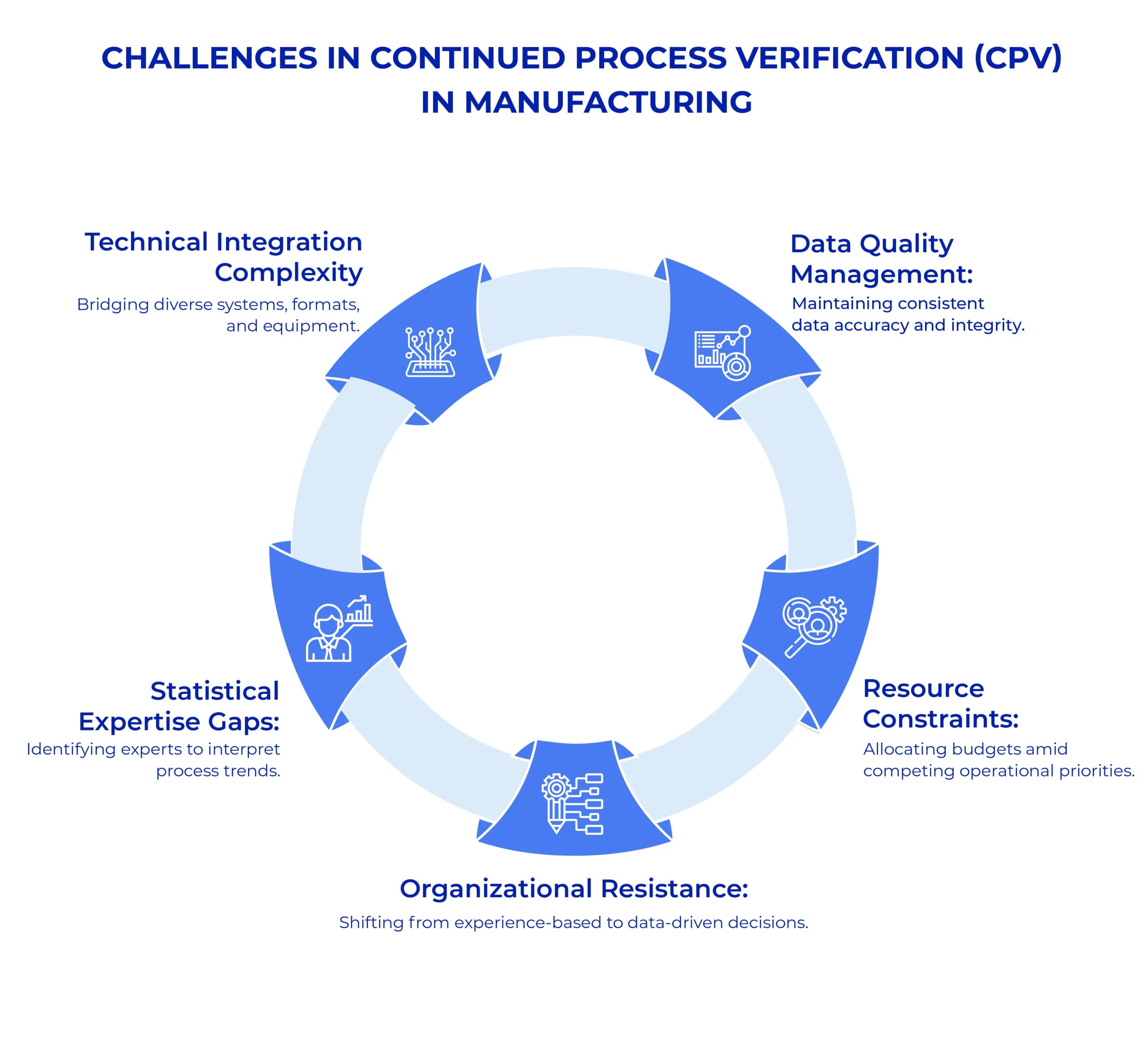
Implementing Continued Process Verification systems in pharmaceutical manufacturing presents multiple obstacles despite its clear benefits for quality assurance and regulatory compliance. Organisations typically encounter resource constraints, technical integration difficulties, and internal resistance when establishing effective CPV programs. These challenges can significantly delay implementation timelines and compromise the effectiveness of verification efforts if not adequately addressed through strategic planning and organizational commitment.
- Technical Integration Complexity: Connecting disparate manufacturing systems, legacy equipment, and various data formats into a cohesive monitoring framework
- Data Quality Management: Ensuring consistent data integrity across thousands of collection points while addressing measurement variations and potential recording errors
- Statistical Expertise Gaps: Finding qualified personnel who can properly interpret trend analyses and distinguish between normal process variations and actual deviations
- Resource Constraints: Balancing the substantial financial investment in software, infrastructure, and training against other operational priorities
- Organizational Resistance: Overcoming traditional manufacturing cultures that resist transitioning from experience-based quality assessments to data-driven decision models
Note: Some of the other challenges that you might want to know are – 7 Challenges in Conducting APQR and How to Overcome Them
The Need for a CPV Platform in Manufacturing
Pharmaceutical manufacturing relies on multiple data sources, including:
- IPQA: Monitoring real-time environmental and operational conditions such as temperature, pressure, and humidity. The CPV platform gathers data in real time via connections to manufacturing execution systems (MES), APIs, and even spreadsheets if needed.
- QC Labs: Analyzing product quality attributes, including purity, potency, and stability.
- Raw Material Data: This includes details about your suppliers, whether a batch of raw materials was accepted or rejected, any impurities found, and certificates of analysis (COAs). The system can connect directly to your ERP system (like SAP or Oracle), pull data from supplier portals using APIs, or import spreadsheets.
However, these data points often exist in isolated systems, making it difficult to identify trends, detect deviations, and ensure consistent process control. A CPV in APQR platform bridges these gaps by integrating, standardizing, and analyzing data to enhance decision-making and regulatory compliance.
How CPV Enhances Data Processing and Storage?
A well-implemented CPV system does more than just collect data, it transforms raw manufacturing data into structured, validated insights.
- Data Validation: Ensuring accuracy, completeness, and reliability of incoming process data.
- Data Normalization: Standardizing measurement units and formats to maintain consistency across systems.
- Secure Data Storage: Organizing structured records for efficient retrieval, trend analysis, and compliance reporting.
Also Read – A Step-by-Step Guide to Process Validation in Pharma
Real-Time Insights with CPV Dashboards
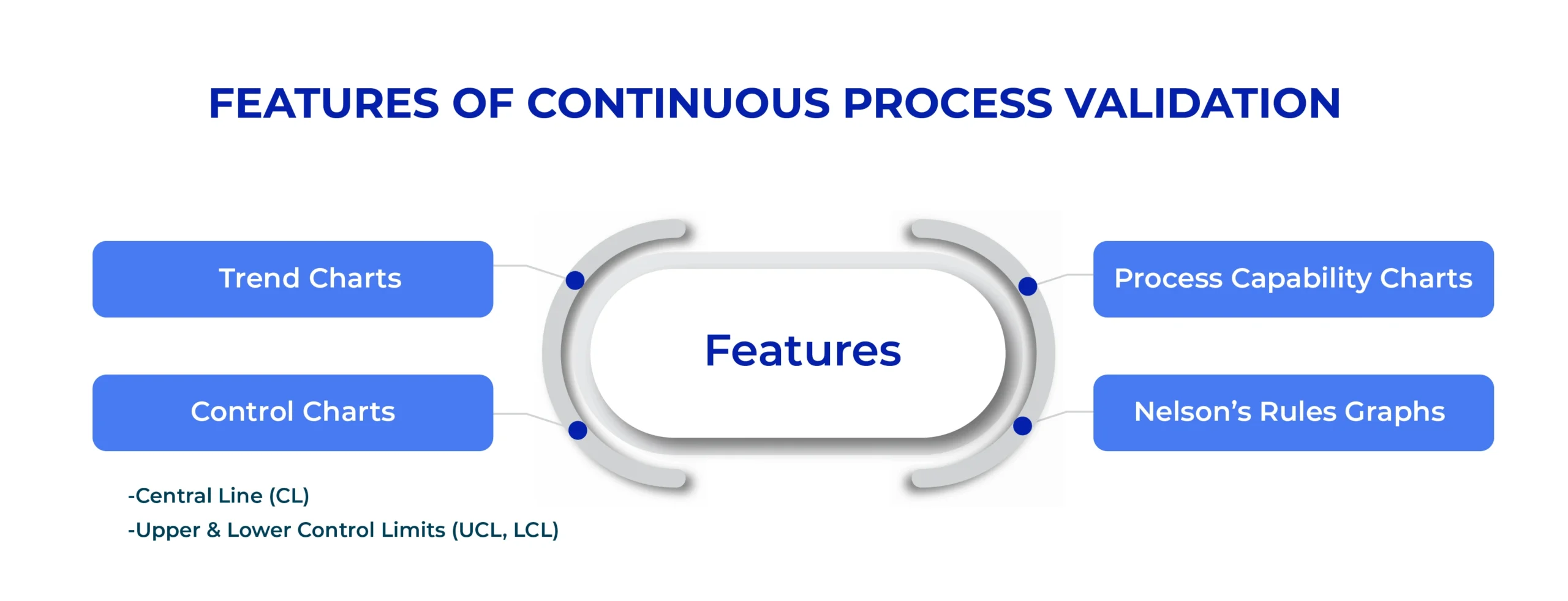
Traditional process monitoring relied on spreadsheets and periodic reviews. Today’s CPV in APQR platforms leverage advanced dashboards to provide real-time visualization of manufacturing performance.
Key Features:
- Trend Charts: Monitoring fluctuations in CQA and CPP across different batches.
- Control Charts: Identifying variations in process stability and distinguishing between normal and abnormal trends.
- Central Line (CL): Average value of the process.
- Upper & Lower Control Limits (UCL, LCL): Define acceptable process variation.
- Process Capability Charts: Evaluating consistency and efficiency through Cp and Cpk indices.
- Nelson’s Rules Graphs: Detecting early warning signs of deviations before they escalate into compliance issues.
Must Read: Exploring the 3 W’s of APQR: What, Why, and Where
Regulatory Compliance: Meeting Global Standards with CPV
Regulatory inspections can be daunting, with changing requirements and tight deadlines. However, a well-implemented CPV in APQR system can serve as a strong defense against these challenges. Regulatory bodies like the FDA, EMA, and ICH Q8-Q10 emphasize ongoing process verification as part of a robust Quality Management System (QMS). A CPV system strengthens compliance by:
- Automating Reports for Audits: Generating instant reports for regulatory inspections.
- Ensuring Data Integrity: Implementing encryption, audit trails, and electronic signatures to maintain record authenticity.
- Real-Time Monitoring: Providing continuous oversight to align with evolving compliance requirements.
Conclusion
In an era of stringent regulatory oversight and increasing product complexity, Continued Process Verification (CPV) is an essential strategy for pharmaceutical manufacturers. Platforms like AmpleLogic’s CPV dashboard empower organizations to harness real-time data, optimize production processes, and mitigate compliance risks effectively.
With AmpleLogic’s CPV in APQR, pharmaceutical manufacturers gain a powerful strategic asset to achieve consistent product quality, optimise processes, and confidently navigate complex regulatory guidelines. Explore AmpleLogic today to see how it can transform your APQR experience. Also, you can visit our page for other such informative articles – AmpleLogic Resources!

About Rajshree Lahoty
Meet Rajshree Lahoty, the writer behind insightful blogs and articles. Armed with a pen mightier than the sword (and a keyboard!), she navigates through the lanes of knowledge with a dash of research and a sprinkle of information.

















