
A lot of essential tasks are carried forward in pharma laboratories to ensure product safety, quality, and compliance. These tasks majorly include pharma research and development, stability testing, post-market surveillance, and so on. Ensuring compliance with stringent and evolving pharma regulations is a mandate in pharma lab operations. This path, however, is laid with numerous challenges like management of complex laboratory data and documentation, and adaption of new regulatory standards.
In this article, we will explore how labs play an integral role in the pharmaceutical industry, the tasks that are carried out in the facility and the compliance challenges it faces. We will further explore how overcoming these hurdles is essential for maintaining integrity, avoiding penalties, and ensuring continued success in the regulated environment. Finally, we will propose a solution that helps tackle these compliance risks. So, lets delve in!
Integral Role of Laboratories in Pharma
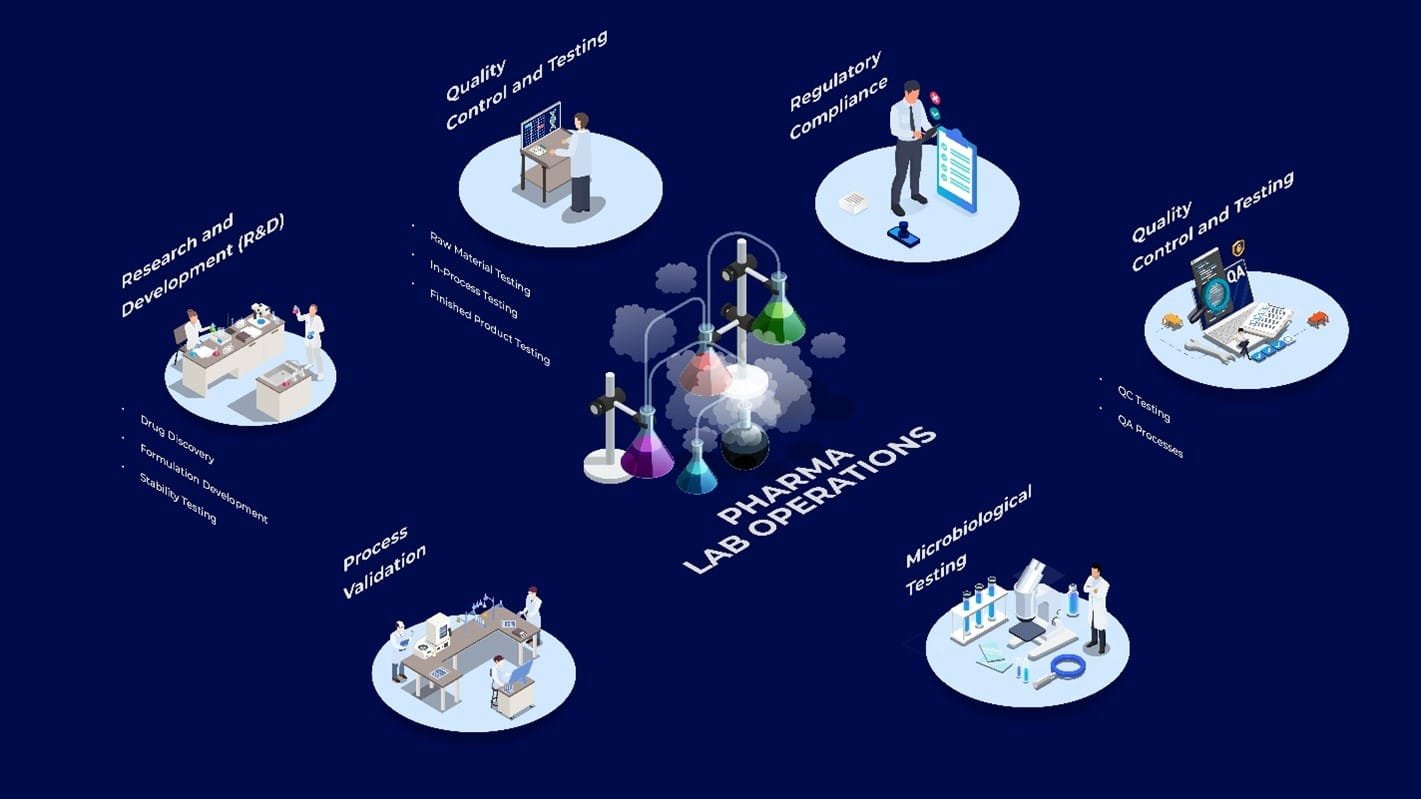
Pharmaceutical laboratories play a crucial role in drug development lifecycle, ensuring regulatory compliance in labs, and quality and safety at every stage—from research and development (R&D) to post-market surveillance. In the R&D phase, labs engage in drug discovery by screening chemical compounds and conducting preclinical studies to assess efficacy and safety, followed by formulation development to optimize dosage forms.
Stability testing is performed to determine the shelf life of drug formulations under various environmental conditions. During the manufacturing phase, labs are involved in raw material testing, in-process testing for consistency and compliance with Good Manufacturing Practices (GMP) and finished product testing to ensure it meets quality and safety standards. Microbiological testing ensures sterility and contamination-free products, especially for injectables.
Regulatory compliance is a key focus, with labs adhering to Good Laboratory Practices (GLP) and FDA regulations, generating essential documentation like Certificates of Analysis (CoAs) and validation reports. Quality Control (QC) labs ensure products meet specifications through chemical, physical, and microbiological testing, while Quality Assurance (QA) ensures compliance with SOPs and regulatory standards. Process validation in labs ensures consistency in drug production, and post-market pharmacovigilance monitors the safety and efficacy of drugs once they are in the market.
Top Compliance Challenges in Pharma Laboratories
Amidst the top challenges faced in pharma laboratories, these stand out as major compliance risks.
Managing Regulatory Changes: Industries like pharmaceuticals must constantly adapt to evolving regulations, including Good Laboratory Practices (GLP), FDA 21 CFR Part 11, and ISO standards. Failure to comply with these guidelines can result in audits, fines, or loss of certification. Tracking and implementing regulatory updates manually often lead to gaps and inefficiencies.
Ensuring Laboratory Data Integrity: Inaccurate, incomplete, or unprotected data can compromise both compliance and results. Manual processes and scattered records make data integrity maintenance difficult. Unauthorized access or lack of robust audit trails further heightens the risk of non-compliance.
Documentation and Reporting: Labs must generate extensive and error-free documentation, such as Certificates of Analysis (CoA), validation reports, and test records for regulatory compliance in labs. Manual report preparation is time-consuming, error-prone, and may fail to meet strict submission deadlines.
Sample Management and Traceability: Complete traceability of samples—from receipt to disposal—is critical. Mismanagement can lead to lost samples, incomplete data, or failed audits. Maintaining proper storage conditions and ensuring accurate labelling are significant challenges, particularly in high-throughput labs.
Managing Resource Efficiency: Compliance-related tasks consume considerable time and resources, often diverting focus from core activities like testing and research. Manual workflows for audits, documentation, and quality checks overburden staff, leading to inefficiencies and potential errors.
LIMS Coming to the Rescue!
Introducing Pharma LIMS Software
Laboratory Information Management System (LIMS) is a specialized lab compliance software solution that streamlines and manages laboratory operations, workflows, and data, enabling industries such as pharmaceuticals, biotechnology, food safety, and environmental testing to operate with greater efficiency, accuracy, and compliance.
Uses of Pharma LIMS Application
LIMS excels in sample management by tracking samples from receipt to disposal, ensuring complete traceability and reliable data for audits. Its data management capabilities centralize information storage, maintaining security and compliance with laboratory data integrity standards like ALCOA+. By enabling workflow automation, LIMS reduces manual tasks, minimizing errors and saving time, which enhances operational efficiency.
LIMS enhances regulatory compliance in labs, through audit trails, electronic signatures, and adherence to FDA 21 CFR Part 11, GLP, and ISO standards. Additionally, it simplifies the generation of critical documents like Certificates of Analysis (CoAs) with customizable templates. LIMS lab compliance software also supports system integration, seamlessly connecting laboratory instruments and enterprise resource planning (ERP) systems to create a unified, efficient workflow.
How LIMS Solves Key Laboratory Challenges
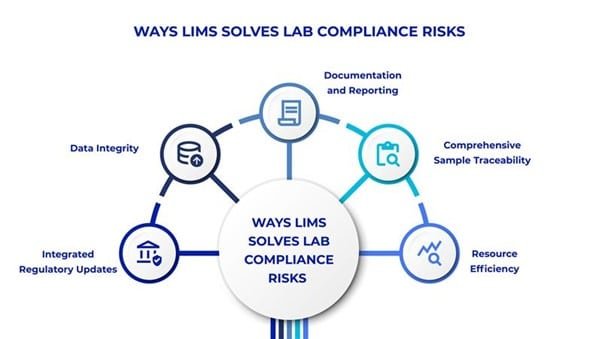
- Use LIMS for compliance: Ensure regulatory compliance in labs with LIMS lab compliance software that seamlessly integrates regulatory requirements, such as FDA 21 CFR Part 11, GLP, and ISO standards into the system, ensuring laboratories stay up-to-date with evolving guidelines. Automated workflows and rule-based compliance checks minimize the risk of errors, while built-in audit trails provide a transparent record to demonstrate adherence during inspections and audits.
- Ensures Laboratory Data Integrity: Centralize all laboratory data into a secure and accessible repository, reducing the risks associated with scattered or incomplete records by using LIMS for compliance. Advanced features like electronic signatures, role-based access controls, and real-time tracking prevent unauthorized changes and ensure data security. Additionally, adherence to ALCOA+ principles strengthen data accuracy, reliability, and traceability.
- Streamlines Documentation and Reporting: Generate essential documents such as Certificates of Analysis (CoAs), batch records, and validation reports using LIMS for compliance reporting. Customizable templates ensure that these documents meet regulatory requirements, while real-time reporting capabilities streamline submission processes and keep labs audit-ready at all times.
- Enables Comprehensive Sample Traceability: LIMS lab compliance software ensures full traceability of samples by assigning unique identifiers and tracking their lifecycle from receipt to disposal. Barcode integration, storage condition monitoring, and automated alerts help maintain sample integrity and ensure compliance with regulatory standards, reducing the risk of mismanagement or lost data.
- Improves Resource Efficiency: Automate routine and time-intensive tasks, such as data entry, sample tracking, and compliance monitoring for error-free records using LIMS for compliance. The application significantly reduces the workload on laboratory personnel. This frees up resources to focus on critical research and testing activities, enhancing productivity while maintaining high levels of compliance with minimal manual intervention.
Compliance is a cornerstone of laboratory operations, especially in regulated industries. A modern LIMS transforms compliance management, offering automation, transparency, and efficiency. From adapting regulatory changes to ensuring laboratory data integrity and streamlining workflows, LIMS empowers labs to excel in an increasingly regulated environment. By adopting LIMS for compliance in pharma labs, it also enhances operational performance, ensuring sustained success while staying ahead of evolving industry standards.





















