
Laboratory Information Management System (LIMS) validation ensures that a LIMS system is fit for its intended purpose, meets the required specifications, and consistently performs as expected. It is achieved by testing and verifying that the LIMS operates according to predefined acceptance criteria, regulatory standards, and user requirements.
The primary objective of LIMS validation is to ensure the system’s correct and accurate functioning, compliance with regulatory industry standards, suitability for its intended use, and the reliability of the data it manages. A validated LIMS system leaves no room for error, ensuring top-notch performance.
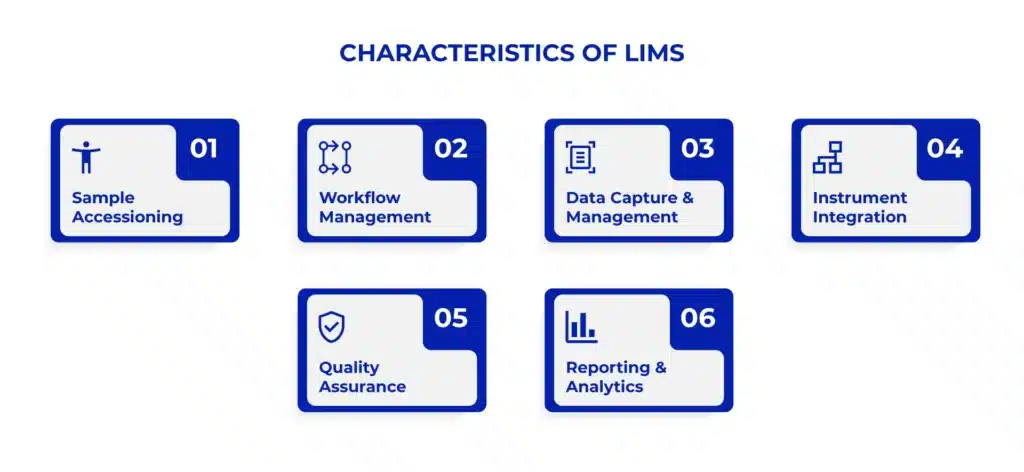
Characteristics of a LIMS System
Sample Accessioning: When a sample is received in the lab, the LIMS assigns it a unique identifier, such as a barcode or tracking number. It allows the sample to be tracked throughout its lifecycle in the lab.
Workflow Management: The LIMS system automates and streamlines laboratory workflows. It can assign samples to the appropriate tests or analyses, schedule the work, and monitor each sample’s status as it progresses. Thus, the LIMS system helps ensure efficient sample processing and reduces the risk of errors.
Data Capture and Management: As the sample is analysed, the LIMS software captures all relevant data, such as test results, instrument readings, and observations. LIMS system data is stored in a centralized database, making it easily accessible for reporting, review, and further analysis.
Instrument Integration: LIMS integration can be done directly with laboratory instruments, such as analytical equipment. It allows data to be transferred automatically from the instruments to the LIMS, eliminating the need for manual data entry and improving data accuracy.
Quality Assurance: LIMS software often includes features supporting quality assurance, such as defining standard operating procedures, setting up quality control checks, and generating audit trails. These systems help laboratories maintain compliance with regulatory requirements.
Reporting and Analytics: LIMS systems provide robust reporting capabilities, allowing users to generate various reports, from simple sample status updates to complex data analyses. Users can use this information to make data-driven decisions and optimize laboratory operations.
Critical Aspects of Validated LIMS Systems
- Performing functional testing to ensure accurate and reliable system features and workflow performance
- Conducting performance and load testing to assess the LIMS’s capability to handle anticipated workloads
- Verifying data integrity, security, and compliance with regulatory requirements such as GLP, GMP, and data integrity guidelines
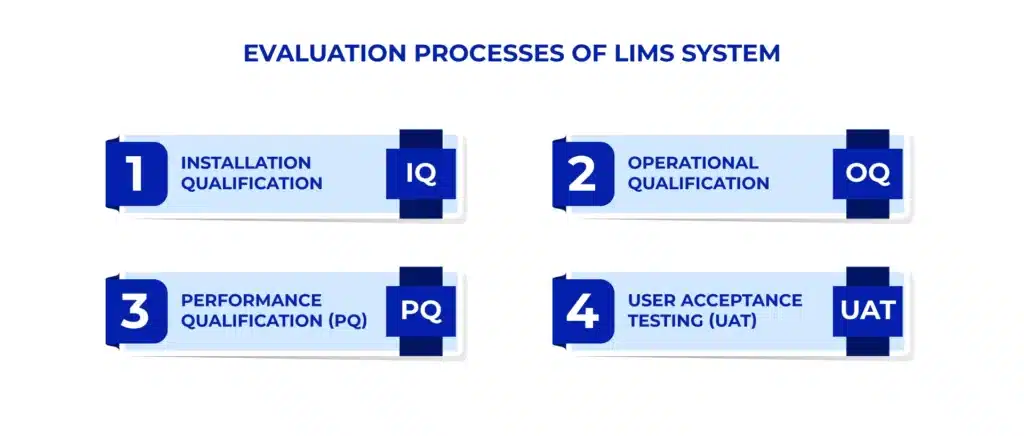
Evaluation Processes of a LIMS System
LIMS validation happens with a series of tests and procedures that evaluates the system’s functionality, performance, and security. These tests include:
- Installation Qualification (IQ): Ensures that the LIMS has been installed correctly
- Operational Qualification (OQ): The LIMS system operates within the specified limits
- Performance Qualification (PQ): Verifies that the LIMS performs consistently and accurately
- User Acceptance Testing (UAT): Involves testing the LIMS with real users to ensure their needs and expectations are met.
Importance of Validated LIMS System in Pharma
Validated Laboratory Information Management System complies with specific requirements and is suitable for the intended use. Ensuring that a LIMS system is reliable, accurate, and produces consistent results is necessary. Validated LIMS System prevents errors, reduces rework, and saves time and resources.
Here are some key reasons why a validated Laboratory Information Management System is important:
- Regulatory Compliance: Pharmaceutical companies must adhere to strict regulations such as Good Laboratory Practices (GLP) and Good Manufacturing Practices (GMP). A validated LIMS ensures that the system meets these regulatory requirements, which is essential for passing audits from regulatory bodies like the FDA.
- Data Integrity and Accuracy: Validation processes confirm that the LIMS accurately captures, stores, and retrieves data. This is vital for maintaining the integrity of laboratory results, which can impact product quality and safety. Errors in data management can lead to significant compliance issues and financial losses.
- Traceability: A validated LIMS provides a clear audit trail of all data entries and modifications, which is critical for tracking the lifecycle of samples and results. This traceability is essential during regulatory audits and for ensuring accountability in laboratory processes.
- Operational Efficiency: By validating a LIMS, pharmaceutical companies can streamline laboratory workflows, reduce errors, and enhance productivity. The validation process helps identify and mitigate potential risks, ensuring that the LIMS operates as intended under real-world conditions.
- Cost-Effectiveness: Proper validation before implementation can save costs associated with post-release modifications and downtime. Identifying issues early in the validation process prevents disruptions in laboratory operations and avoids costly rework.
- Enhanced Security: Validation includes testing the LIMS for data protection against unauthorized access and modifications. This is particularly important in the pharmaceutical industry, where sensitive data is handled regularly.
- User Confidence and Training: A validated system boosts user confidence in the LIMS capabilities. It ensures that end-users are adequately trained on a system that meets their operational needs and complies with regulatory standards, further enhancing productivity and compliance.
LIMS validation is a critical process that ensures a Laboratory Information Management System meets its intended purpose and regulatory requirements. It guarantees data integrity, accuracy, and reliability, which are essential in a laboratory setting. LIMS software validation prevents errors, reduces rework, and saves time and resources. LIMS software ensures compliance with industry standards and regulatory requirements. A validated LIMS provides a complete audit trail, quality control, and data security. Laboratories need to implement a validated LIMS to ensure the quality and reliability of their testing results. By doing so, laboratories can maintain regulatory compliance, reduce errors, and improve overall efficiency. Ultimately, LIMS validation is crucial for ensuring the accuracy and reliability of laboratory test results.




























