Pharmaceutical Production Stability Issues: Leveraging MES for Real-Time Monitoring and Remediation

Stability issues present significant challenges in the realm of pharmaceutical manufacturing threatening product quality, regulatory compliance, and ultimately patient safety. Traditional approaches to monitoring and managing these issues often fall short, hindered by manual interventions, inefficient processes, and compliance complexities. The integration of Manufacturing Execution Systems (MES) for pharma manufacturing offers a transformative remedy, enabling real-time monitoring and proactive remediation to ensure stability throughout the production lifecycle.
This article aims to enhance your understanding of the challenges related to stability issues in pharmaceutical production. It delves into how AmpleLogic, a leading provider of GAMP solutions, enhances Manufacturing Execution Systems (MES) for pharmaceutical manufacturing to effectively tackle these challenges and bridge existing gaps. Explore further with us!
Current Challenges in Pharmaceutical Production Stability
Lack of Real-Time Monitoring: Traditional methods rely on manual interventions and periodic inspections, leading to delays in identifying stability issues.
Inefficient Production Planning and Scheduling: Manual processes and outdated systems struggle to adapt to dynamic production demands, resulting in inefficient resource allocation.
Compliance and Traceability Challenges: Manual documentation processes and fragmented data systems make it challenging to achieve comprehensive traceability and regulatory adherence.
Inefficient Resource Utilization and Lean Manufacturing: Manual workflows inhibit the implementation of lean manufacturing principles, resulting in waste and inefficiencies.
Implementing AmpleLogic MES for Pharmaceutical Manufacturing
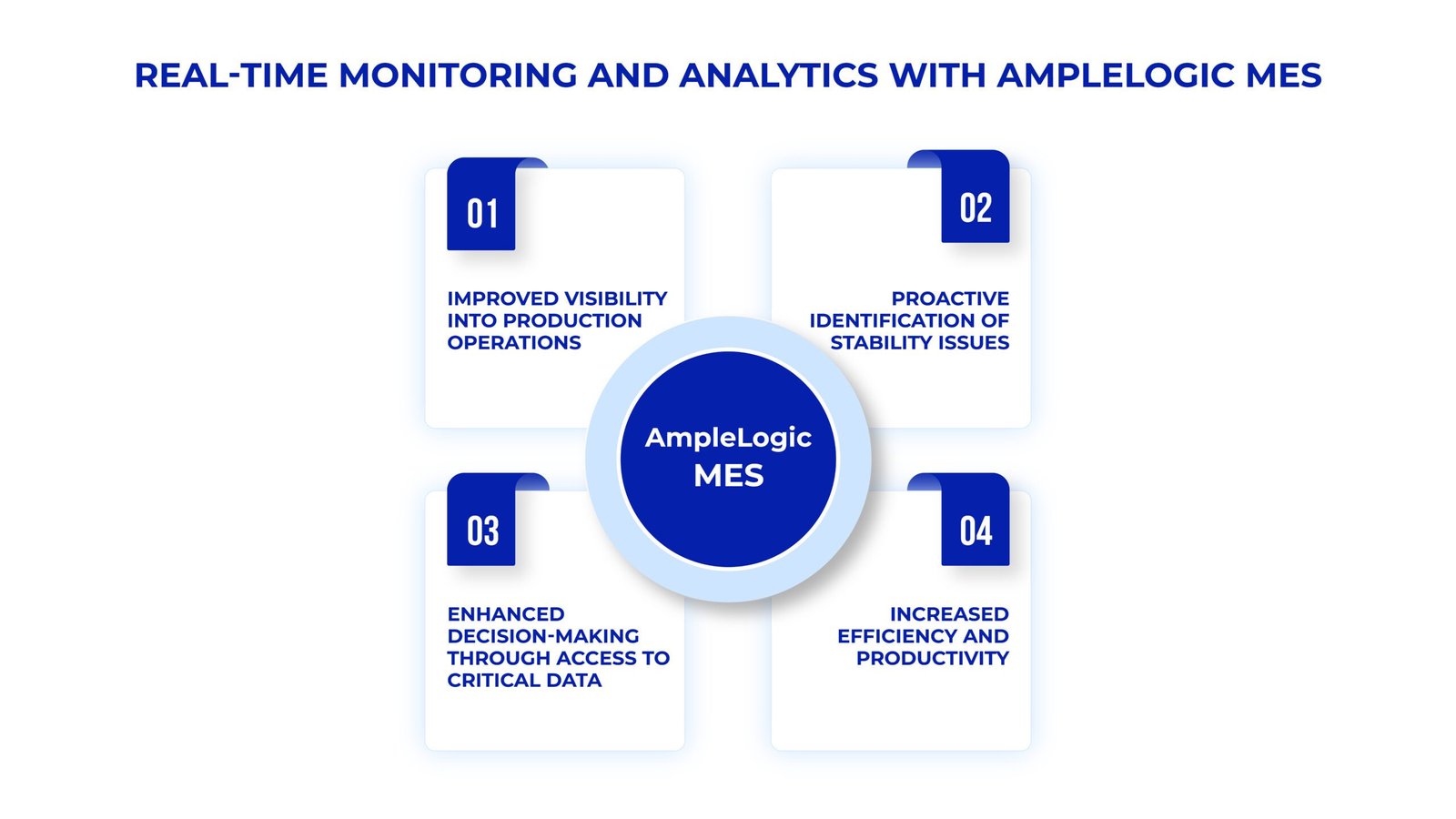
1. Real-Time Monitoring and Analytics
MES for Pharmaceutical Manufacturing: Implementing AmpleLogic MES enables real-time visibility into production operations, facilitating proactive identification of stability issues.
MES Monitoring System: AmpleLogic MES provides stakeholders with access to critical data and analytics dashboards for prompt decision-making and intervention.
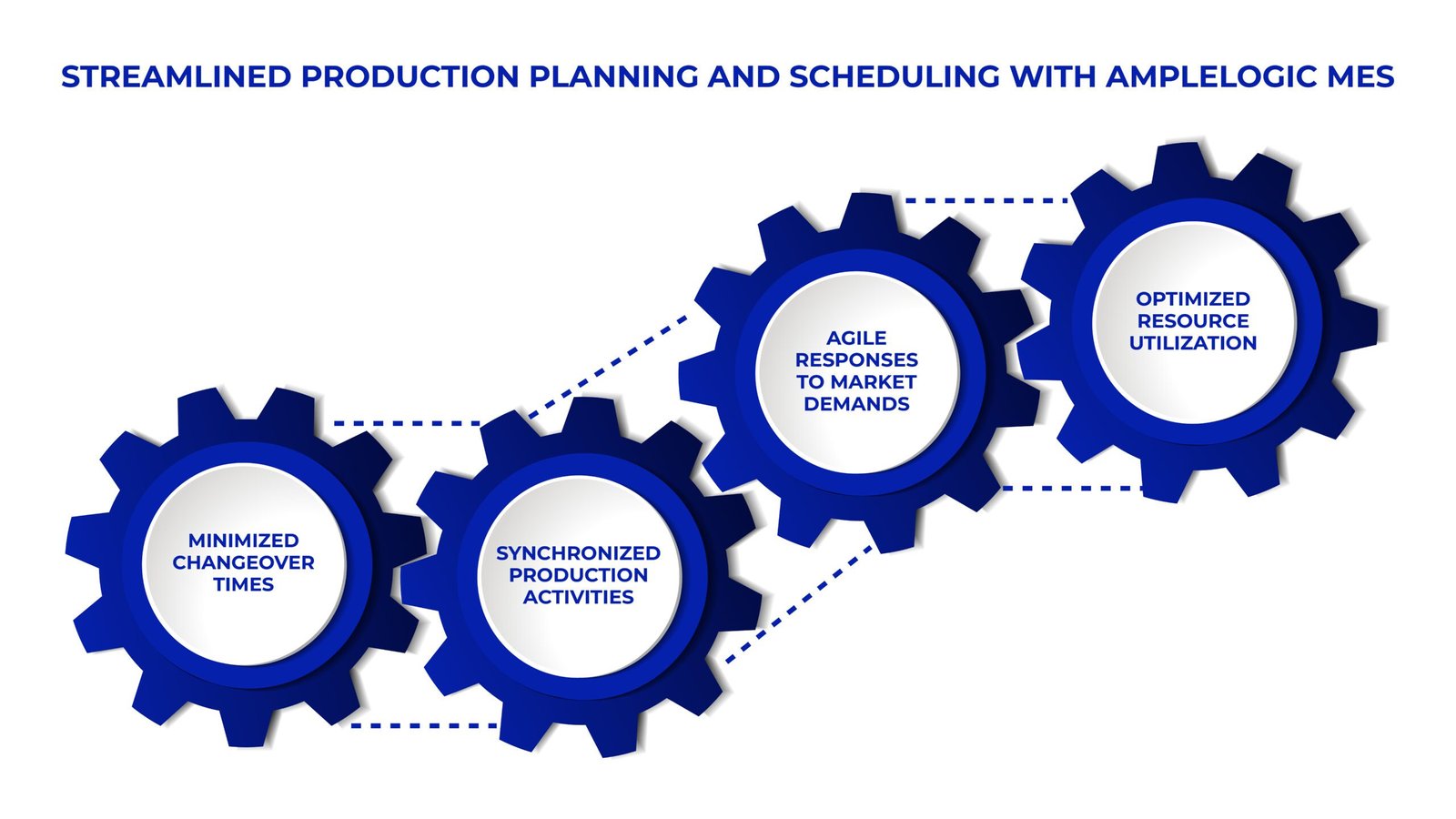
2. Streamlined Production Planning and Scheduling
MES for Production Planning and Scheduling: AmpleLogic MES offers advanced capabilities to optimize resource utilization, minimize changeover times, and synchronize production activities.
Manufacturing Tracking Software: Automated scheduling algorithms and real-time production tracking enable agile responses to changing market demands.
3. Enhanced Compliance and Traceability
MES Traceability System: AmpleLogic MES offers a paperless solution to compliance and traceability challenges, digitizing documentation processes and implementing robust traceability systems.
MES Software for Compliance: Ensuring every manufacturing step is documented and traceable, AmpleLogic MES expedites audit readiness and regulatory compliance.
4. Lean Manufacturing and Operational Excellence
MES for Lean Manufacturing: AmpleLogic MES integrates lean manufacturing principles into pharmaceutical production processes, optimizing resource utilization and driving continuous improvement.
MES Lean Manufacturing Solutions: By standardizing workflows, reducing cycle times, and implementing just-in-time production strategies, AmpleLogic MES facilitates operational excellence and stability.

In addressing pharmaceutical production stability issues, the implementation of AmpleLogic MES emerges as a comprehensive solution. By offering real-time monitoring, streamlined production processes, enhanced compliance, and lean manufacturing principles, AmpleLogic MES empowers pharmaceutical manufacturers to navigate industry challenges effectively and ensure sustainable stability in their operations.





























