Integrating QMS with ERP, LMS, and LIMS Can Boost Compliance and Efficiency by 70% in 2025

What is a Pharmaceutical Quality Management System (QMS)?
A Pharmaceutical Quality Management System (QMS) is a structured framework of policies, procedures, and processes designed to ensure that pharmaceutical products are manufactured to meet high-quality standards and comply with industry regulations. It is essential for maintaining consistent product quality, safety, and efficacy.
Critical components of a Pharmaceutical QMS include:
- Document Management: Managing policies, procedures, and records with explicit version control.
- Change Control: Documenting and controlling changes to prevent errors or risks.
- Training Management: Ensuring personnel is trained on quality standards and procedures.
- Audit Management: Conduct regular audits to ensure adherence to the system.
- CAPA Management (Corrective and Preventive Actions): Addressing non-conformances and preventing future occurrences.
- Deviation Management: Identifying and managing deviations from standard procedures.
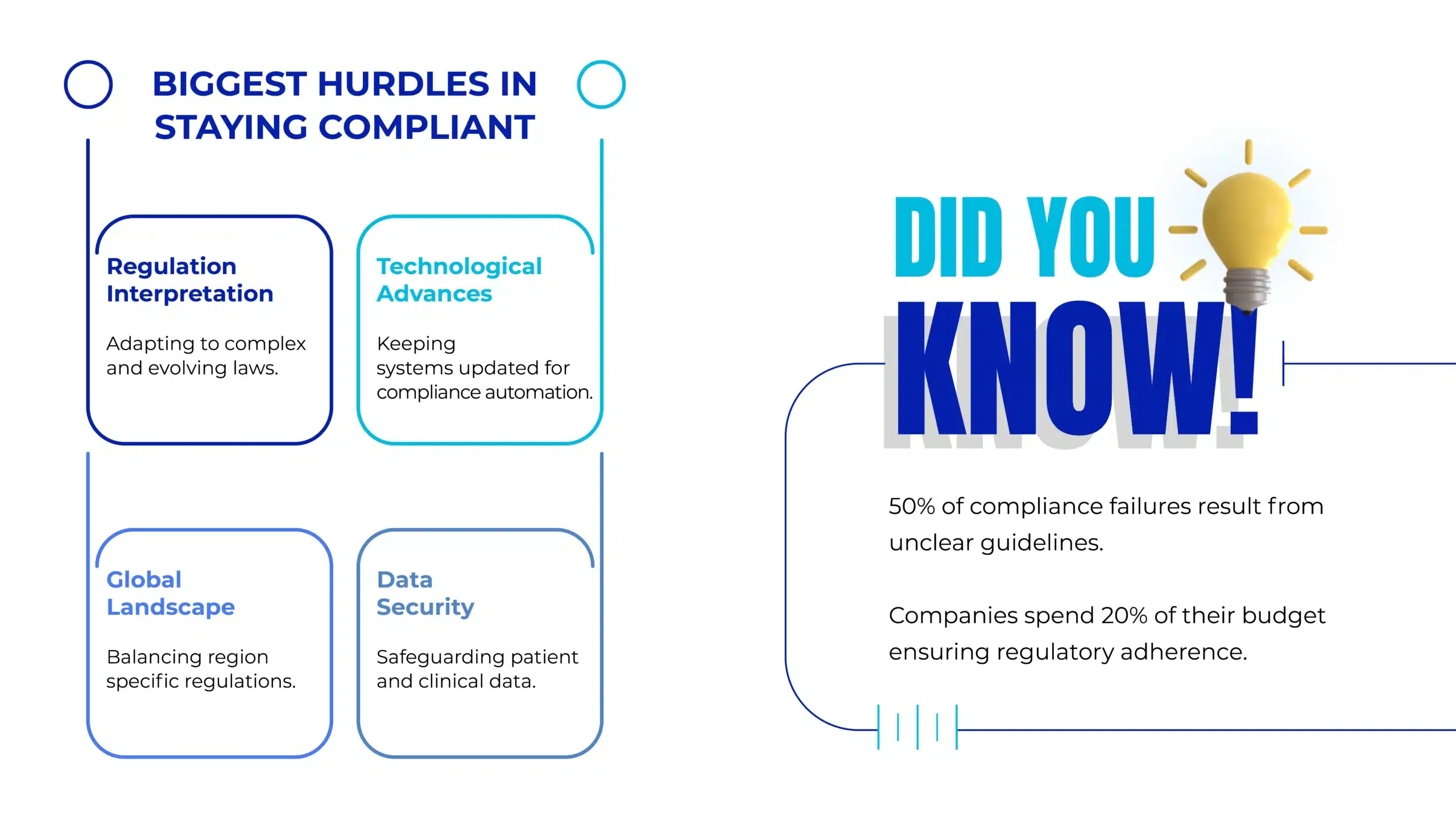
Regulatory Requirements for Pharmaceutical QMS
Pharmaceutical companies must implement a QMS that aligns with various international regulations and standards to ensure compliance and product safety. Some of the most critical regulations include:
International Organization for Standardization (ISO)
ISO develops standards for various industries, including pharmaceuticals. The current QMS standard is ISO 9001:2015, which helps pharmaceutical companies improve their performance and ensure compliance with quality management requirements. It’s widely used to streamline processes, reduce errors, and provide customer satisfaction.
Pharmaceutical Inspection Co-operation Scheme (PIC/S)
PIC/S is a collaborative group of global regulatory authorities focused on Good Manufacturing Practice (GMP). It publishes guidance documents such as the PIC/S GMP Guides, which are critical for pharma companies in developing QMS. These guidelines align with global GMP regulations like the EudraLex Volume 4 and cover essential areas like personnel, documentation, quality control, and product recall procedures.
ICH Guideline Q10
The International Council for Harmonization (ICH) sets global pharmaceutical standards. Q10 focuses on the Pharmaceutical Quality System, consolidating best practices, including ISO standards and cGMP guidelines. It integrates quality risk management and pharmaceutical development to ensure products meet safety and efficacy standards. The role of an electronic QMS (eQMS) in achieving compliance with ICH Q10 is crucial for simplifying and improving quality processes.
Current Good Manufacturing Practice (cGMP)
cGMP regulations, enforced by the FDA, ensure safe, effective, and high-quality pharmaceutical products are produced. Compliance is mandatory for pharmaceutical companies to market products, with inspections focusing on quality control, manufacturing processes, and facility conditions. Non-compliance can lead to penalties or product recalls.
FDA 21 CFR Part 210 & 211
These regulations specify cGMP requirements for drug manufacturing and ensure that products are safe and meet the necessary standards. Part 210 covers drug processing and packaging, while Part 211 focuses on finished pharmaceuticals. Compliance with these regulations is essential for maintaining product integrity and safety.
FDA 21 CFR Part 11
This regulation governs the pharmaceutical industry’s use of electronic records and electronic signatures. It ensures digital documentation’s authenticity, integrity, and security, which is vital for maintaining regulatory compliance.
EU GMP Annex 11
Annex 11 provides guidelines for computerized systems in pharmaceutical manufacturing. It focuses on ensuring that digital tools do not compromise product quality, process control, or overall safety. It also covers aspects like data storage, security, and electronic signatures.
ISPE GAMP5
The Good Automated Manufacturing Practice (GAMP5) guidelines, developed by ISPE, provide best practices for computerized systems in pharma. The goal is to ensure these systems meet compliance standards while being cost-effective and suitable for their intended use. GAMP5 ensures that the software used in QMS meets FDA and GMP regulations.
Why Integrate QMS with Existing Systems?
Integrating QMS with existing systems within the organization leads to better control, fewer errors, and a more efficient workflow. Integration eliminates silos of data, fosters collaboration, and enhances visibility across departments, ensuring compliance and reducing the risk of errors.
Here are the key benefits of integrating QMS with existing systems:
- Streamlined Data Flow: QMS integration with systems like ERP, MES, and DMS ensures seamless data sharing and a unified platform for decision-making.
- Reduced Redundancy: Data duplication is minimized as systems share critical information automatically, reducing the need for manual data entry.
- Increased Compliance: Integrated systems can automatically generate audit trails, making it easier for regulatory bodies to track compliance and ensure adherence to standards.
- Enhanced Efficiency: Automating audit management, CAPA, and deviation reporting reduces manual intervention, allowing employees to focus on high-value tasks.
- Real-time Reporting: Integration allows real-time monitoring and reporting of key quality metrics, helping pharmaceutical companies respond proactively to issues before they escalate.
Key Integrations to Consider for QMS
Discover how integrating these key systems led to a 70% boost in efficiency and compliance, transforming pharmaceutical operations and elevating overall quality management. Check out the detailed breakdown below:
1. Learning Management Systems (LMS)
An LMS is critical for ensuring employees are trained and compliant with quality standards. Integrating LMS with QMS allows for automated tracking of training completion and compliance records, ensuring that all employees are up-to-date with the latest quality procedures and regulations.
2. Document Management Systems (DMS)
DMS systems manage the documentation and record-keeping that support compliance and operational efficiency. Integration of QMS with DMS ensures that documents, such as SOPs and quality manuals, are automatically stored, versioned, and accessible for audits. This integration eliminates manual errors in document handling and ensures accurate documentation of all processes.
3. Laboratory Information Management Systems (LIMS)
LIMS plays a crucial role in the pharmaceutical industry by managing samples, tests, and lab workflows. Integrating LIMS with QMS ensures that laboratory data such as test results and analysis are directly transferred into the quality management system. This helps maintain data integrity and ensures that results align with quality standards for compliance.
4. Enterprise Resource Planning (ERP)
ERP systems are designed to handle business operations such as finance, supply chain management, and manufacturing. Integrating QMS with ERP allows for seamless management of materials, inventory, and production schedules while maintaining compliance. By linking the two systems, companies can track production from raw materials to finished products while ensuring product quality at every stage.
5. Manufacturing Execution Systems (MES)
MES systems provide real-time control over manufacturing processes, ensuring products are produced according to established quality standards. By integrating MES with QMS, pharmaceutical companies can ensure that manufacturing data is captured and monitored in the QMS, allowing for timely interventions in case of quality deviations.
6. Electronic Lab Notebooks (ELN)
Integrating QMS with ELN allows researchers and scientists to record experimental data digitally while complying with regulatory standards. This ensures laboratory data is easily traceable, accessible for audits, and connected with other quality management processes.
7. Annual Product Quality Review (APQR)
APQR is an essential part of a pharmaceutical company’s ongoing quality assurance. By integrating QMS with APQR systems, companies can automate the process of reviewing product quality over time, ensuring that products continue to meet regulatory and customer standards.
These 7 integrations reduce human errors, enhance decision-making, and accelerate processes across departments—leading to a 70% compliance and operational efficiency improvement by 2025!
Best Practices for Successful Integration
To ensure the successful integration of QMS with existing systems, consider the following best practices:
- Identify Key Systems for Integration: Assess your existing systems and determine which ones are crucial for quality management. Prioritize integration with ERP, MES, and LIMS for maximum impact.
- Data Standardization: Ensure data formats and structures are standardized across all systems to avoid discrepancies during integration.
- Choose Scalable Solutions: Select QMS solutions that can scale as your business grows and easily integrate with future technologies and systems.
- Training and Support: Train employees to use the integrated systems effectively, ensuring smooth adoption and usage.
- Continuous Monitoring and Improvement: Continuously monitor system performance and identify areas for improvement to optimize integration and streamline processes.
Role of Amplelogic’s eQMS in Integration
Amplelogic’s eQMS offers an integrated platform to facilitate compliance with regulations such as ISO, cGMP, FDA 21 CFR Part 11, and EU GMP Annex 11. With its advanced features, Amplelogic eQMS supports the seamless integration of various systems, including LMS, DMS, LIMS, MES, and ERP, ensuring that all quality management processes are unified.
Here are some features of Amplelogic’s eQMS:
- Document Control: Centralized control of all documents to ensure version control, regulatory compliance, and easy retrieval during audits.
- Training and Compliance: Automated employee training tracking real-time reporting of completion statuses and compliance.
- Audit Management: Streamlined audit planning, execution, and tracking within the system, integrated with CAPA management.
- Supplier Management: Supplier data, including corrective actions and performance evaluations, are integrated directly into the QMS for better supply chain visibility.
- Real-time Data Analytics: Amplelogic’s eQMS offers comprehensive analytics to monitor key quality metrics, identify trends, and drive data-driven decisions.
Integrating Pharmaceutical Quality Management Systems (QMS) with existing systems is vital in today’s evolving pharmaceutical environment. Companies can enhance compliance, improve operational efficiency, and mitigate risks by integrating critical systems such as ERP, MES, LMS, and LIMS with QMS.
With the help of modern eQMS solutions like Amplelogic, pharmaceutical companies can ensure real-time data flow, robust document control, and automated quality management, driving continuous improvement while maintaining the highest quality standards.
Effective integration of QMS with other systems is essential for streamlining quality processes, reducing redundancies, and maintaining regulatory compliance. This approach enhances operational efficiency and ensures that pharmaceutical products are safe, effective, and of the highest quality!





















