
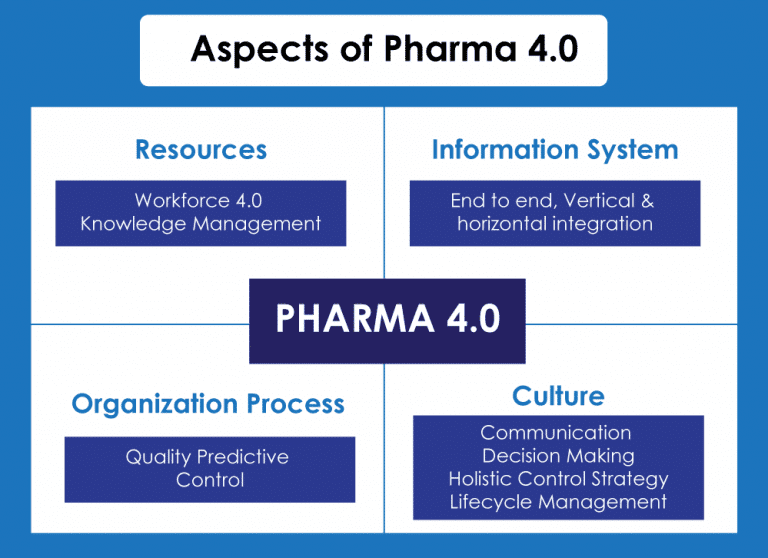
Pharma 4.0 is the pharma, biopharma & life sciences’ response to the fourth industrial revolution, applying digitalization, automation, and advanced data technologies to pharmaceutical manufacturing and lifecycle management. The term “Pharma 4.0” was introduced by the International Society for Pharmaceutical Engineering (ISPE) in 2017 and builds directly on the broader Industry 4.0 initiative started in Germany in 2011.
Unlike previous strides in automation, Pharma 4.0 focuses not only on digitalizing processes but also on establishing a network of integrated systems that ensure real-time decision-making, strong data integrity, and adaptive, patient-centric operations.
The shift responds directly to rising production complexity, increasing compliance requirements, extended time-to-market cycles, and vulnerabilities exposed in traditional supply chains. These demands require infrastructure capable of real-time visibility, cross-functional integration, and continuous regulatory alignment.
Where Traditional Systems Fall Short?
Several persistent challenges in the industry led to the emergence of Pharma 4.0:
- Rising Product Complexity: Biologics, personalized therapies, and variable production lines increased process variance and compliance demands.
- Extended Timelines/Editions: Traditional drug development and manufacturing were slow, with delays straining both innovation and patient access.
- Stringent Regulation: The need for higher traceability and compliance, especially in data integrity and quality management, highlighted the inefficiency of manual and siloed systems.
- Supply Chain Vulnerabilities: Disruptions exposed limitations in legacy systems’ ability to provide end-to-end visibility.
Industry and regulatory bodies recognized that embracing integrated digital models was critical for maintaining product quality, competitiveness, and patient safety.
Stages of Pharma 4.0 Maturity
Organizations advance through clearly defined stages of digital maturity:
- Pre-Digital: Manual and digital/automated elements coexist but are not integrated; data is fragmented.
- Initiated/Basic Digitalization: Introduction of digital technology for repetitive tasks; automated elements grow but largely operate in silos.
- Integrated: Systems and processes connect, eliminating silos. Business and production processes are digitally linked to support better decision-making.
- Harmonized: Organization-wide focus on automation; digital approaches are standard practice; strategic alignment across teams.
- Optimized/Adaptive: End-to-end optimization through real-time data, advanced analytics, and predictive automation. Production adapts in real time with minimal human intervention.
Throughout these stages, organizations move from basic computerization to fully adaptive, intelligent operations. ISPE’s 2023 data shows that 58.1% of pharmaceutical firms are now running both pilot and systematic digital programs, up from 31.2% in 2021.
The Role and Advantages of Low-Code Platforms in Pharma 4.0
Low-code platforms directly address core digital transformation bottlenecks:
- Faster Development: Applications for quality, compliance, and production can be built and validated 30–70% faster than by traditional means.
- Resource Efficiency: Reduces reliance on specialized developers, a growing concern as over 60% of life sciences IT leaders report talent shortages and project backlogs.
- Validation Acceleration: Low-code projects cut validation workloads by as much as 40% through prebuilt compliance toolkits, audit trails, and templated workflows.
- Comprehensive Integration: Native connectors enable seamless integration with LIMS, ERP, MES, and other digital platforms, a necessity for mature digital operations.
- Audit & Compliance Readiness: Automated version control, eSignatures (21 CFR Part 11/EU Annex 11), and immutable audit logs support fast, continuous regulatory inspection.
- Workforce Optimization: Enables non-developers to participate in solution building, reducing reliance on scarce technical resources.
- Real-Time Monitoring: Automated workflows support exception handling and traceability, improving readiness for regulatory inspections.
Adoption Metrics and Market Trends
Pharma 4.0 Market
- Estimated at USD 15.2–19.6 billion in 2025, expected to exceed USD 81–87 billion by 2034 (CAGR 18–19%).
- North America leads with over 43% market share; Asia Pacific is the second-largest region.
Low-Code Platforms
- Valued at USD 37.4–50.3 billion in 2025; projected to grow to USD 157.7–264.4 billion by 2032–34 (CAGR 26.1–33%).
- Pharmaceutical firms cite low-code as essential to shortening deployment times, particularly for regulatory (GxP) processes.
Digital Projects
- In 2025, 55–60% of pharmaceutical companies are engaged in active digital transformation projects, a significant rise from 38% in 2021.
The Bigger Picture: Building Digital Resilience
- The seventh ISPE Pharma 4.0™ Survey (2023) highlights widespread use of AI, machine learning, Industrial IoT, big data analytics, and cloud computing, with these technologies central in manufacturing, supply chain, R&D, and quality functions
- By 2025, leading pharma organizations are deploying automation systems with real-time monitoring, digital twins method, advanced robotics, and predictive analytics, resulting in higher efficiency, reduced human error, and better regulatory compliance.
- Blockchain and IoT technologies are increasingly used for supply chain integrity and laboratory data integrity, reducing counterfeit drugs and ensuring traceability from raw materials to finished products.
- Companies report 30–70% cycle time reductions in GxP workflows, with solution rollouts reduced from months to weeks.
- More than 55% of pharma firms have partially or fully integrated digital technologies by 2025, though full transformation remains a work in progress for many organizations.
Cross-Sector Alignment
Industry organizations such as ISPE continue to expand Pharma 4.0 initiatives through structured knowledge exchange, benchmarking surveys, and capability-building frameworks. Collaborative ecosystems are playing a critical role in standardizing digital maturity benchmarks and compliance expectations globally.
Use Cases Across the Pharma Value Chain
Low-code platforms can transform multiple touchpoints across the pharmaceutical product lifecycle. Here’s how companies are applying them today:
- Quality Management (eQMS): Automate CAPA, deviations, audits, and document control with traceability and centralized dashboards.
- Manufacturing Execution System: Digitalize batch records and enable real-time monitoring of production data.
Regulatory Submissions: Track submission timelines, version history, and compliance tasks with collaborative workflows. - Lab Data Management: Streamline sample tracking, test approvals, and reporting across distributed lab environments with eDMS.
- Training Management: Ensure employees are trained, certified, and audit-ready with integrated learning workflows with LMS.
- Change Control: Digitally log, route, and approve change requests with built-in risk assessments and stakeholder sign-offs.
AmpleLogic: A Low-Code Partner Built for Life Sciences
At AmpleLogic, we specialize in low-code solutions designed specifically for pharma and life sciences enterprises. Our platform comes pre-equipped with industry-specific modules that comply with regulatory standards, including:
- 21 CFR Part 11 compliant e-signatures and immutable audit trails
- Configurable templates for CAPA, deviation, and SOP management
- Secure data handling and validation support
- Real-time dashboards and role-based access control
- Integration with your existing systems and infrastructures, like LIMS, MES, and ERP systems.
Our platform is designed to align with the sector’s compliance frameworks while offering the agility needed to support rapid deployment across quality, laboratory, regulatory, and manufacturing functions.
Conclusion
Pharma 4.0 has evolved from a conceptual model to a structured execution path for regulated life sciences. Organizations are moving steadily across maturity levels, with adoption supported by real-world metrics and operational outcomes.
Low-code platforms have emerged as a practical accelerator. They reduce development and validation timelines, ease IT resource constraints, and provide configurable tools to support compliance at scale. For executive teams tasked with driving operational efficiency, regulatory readiness, and product delivery timelines, low-code adoption offers measurable returns in speed, control, and system adaptability.
For companies aiming to future-proof their operations and navigate regulatory complexity with agility, structured investment in configurable digital infrastructure, such as low-code, is becoming a strategic imperative.





























