
Changing processes in the pharmaceutical industry undergoes a long and redundant change control method. By industry definitions, change control is the systematic way by which regulated sectors such as pharma, initiate, implement and approve process and product changes. This ensures that all changes are documented, evaluated, and implemented systematically to avoid negative impacts on product quality or compliance. The traditional change control process is however inefficient with multi-level initiations and approvals. Let’s delve deeper into these challenges and see how we can essentially eliminate these obstacles.
Let’s Face the Difficulties!
Change management in the pharmaceutical industry undergoes several hurdles that can directly affect processes, hinder quality and regulatory compliance. Despite adoption of electronic change management systems to streamline processes, several challenges persist, leading to inefficiencies and late implementations.
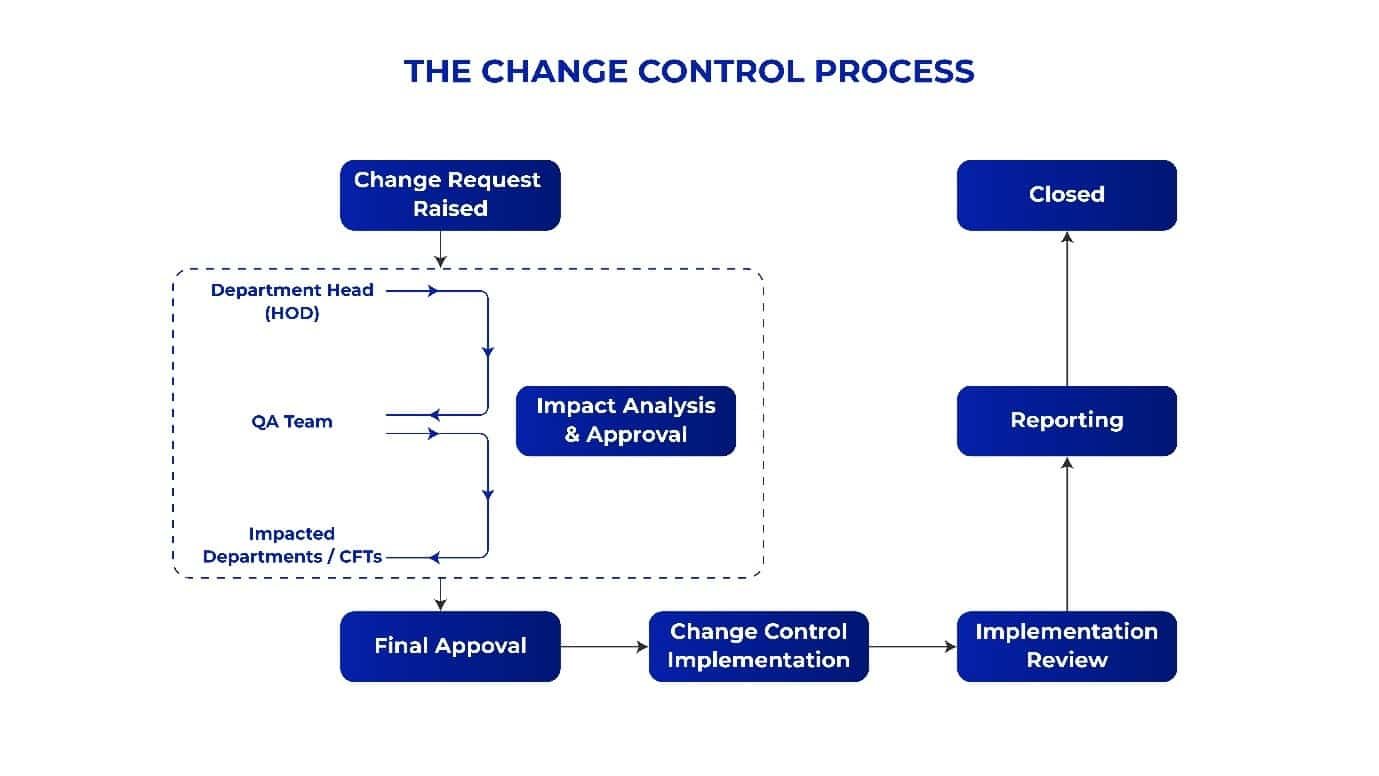
- Extended Approval Cycles:
Change control processes often require multiple layers of approval from Department Heads (HODs), Quality Assurance (QA) teams, and cross-functional teams (CFTs). While this ensures thorough evaluation and compliance, repeated reviews and back-and-forth communications contribute significantly to delays. In some cases, approvals are stuck due to the unavailability of stakeholders or lack of clarity, unnecessarily extending timelines. - Undefined Completion Timelines:
Many change control systems lack clearly defined deadlines for task completion, leading to open-ended processes. Without a structured timeline, tasks are deprioritized or delayed, causing further setbacks in project implementation and compliance adherence. - Lack of Actionable Plans:
A major hurdle is the absence of well-defined, actionable plans for implementing changes. Without clear steps and responsibilities outlined for stakeholders, the process becomes disorganized, leading to miscommunication, redundancies, and inefficiencies. - Inefficient Communication Channels:
Cross-functional coordination is critical in pharmaceutical change management, but ineffective communication between teams often results in missed updates, misunderstandings, and delayed actions. This issue is exacerbated in larger organizations with siloed operations. - Inadequate System Capabilities:
While electronic change control systems aim to simplify processes, many existing solutions lack advanced features such as automated notifications, real-time tracking, or robust analytics. These shortcomings limit their ability to identify bottlenecks and optimize workflows. - Regulatory Demands:
The pharmaceutical industry must comply with stringent regulatory frameworks like FDA’s 21 CFR Part 11, which require meticulous documentation, traceability, and validation for every change. Meeting these standards can be labour-intensive and time-consuming, especially when systems are not fully optimized.
Introducing AI in Pharma Change Control
Artificial Intelligence is an exceptional tool that can transform how we perceive change management process in pharmaceutical industry. When implemented in the right areas, it can bring forth transformative benefits. Here’s a closer look into how we can leverage AI-powered recommendation systems in pharma for cost-effective and faster change control.
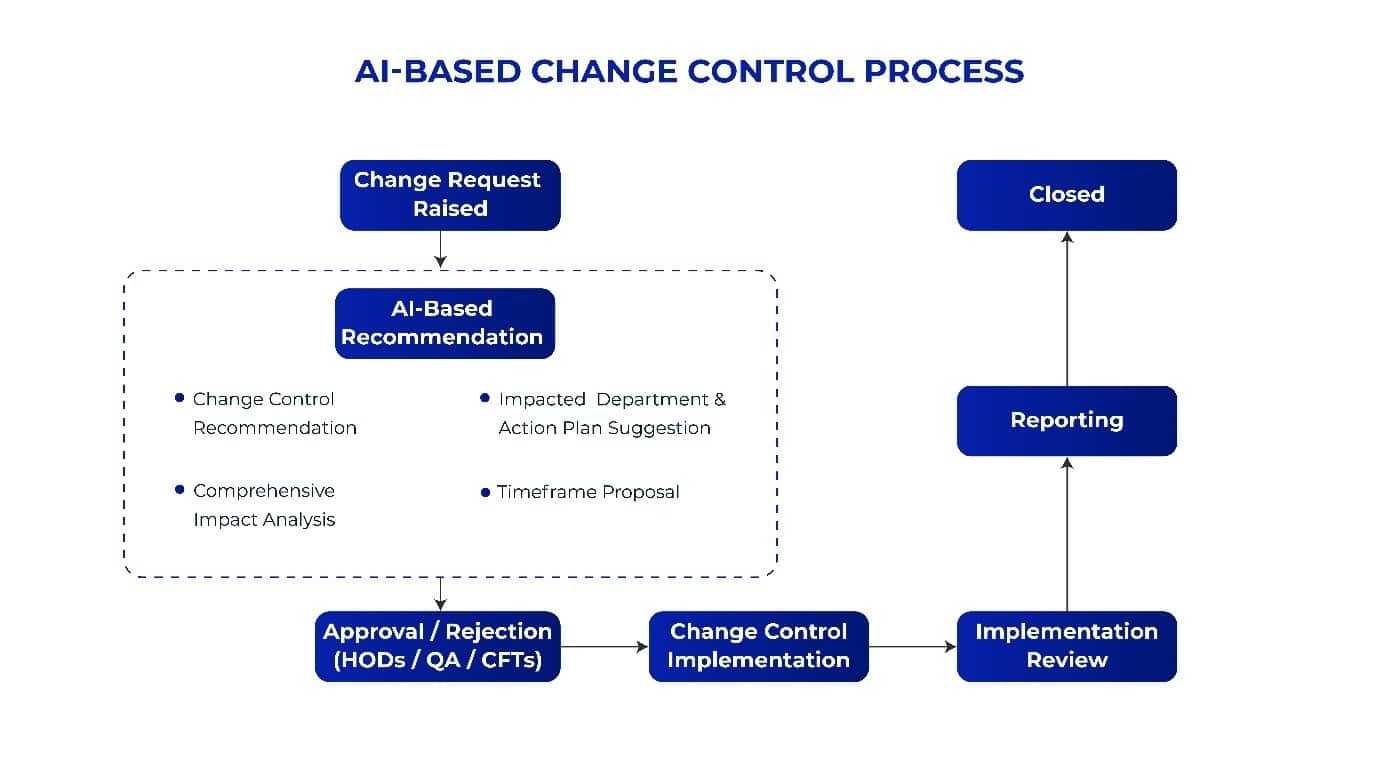
- Change Control Recommendation: In a general scenario, employees need to draft change control management plan for every minute pharma operation or product change, even if a similar situation has occurred earlier and similar change control request was raised earlier. This makes processes redundant, repetitive and time consuming. If AI is implemented at the initiation stage, it can help in risk assessment and provide a list of change control recommendations using the historical data that is already saved in your system. Now you just have to choose from the list of change control measures that is recommended by the AI.
- Impacted Department & Action Plan Suggestion: AI can streamline the change control process by automatically outlining action plans and identifying impacted departments, reducing the workload for Department Heads, the QA team, and cross-functional teams (CFTs). This reduces time and effort, accelerating the execution of pharmaceutical change control.
- Comprehensive Impact Analysis: Through sophisticated algorithms, AI evaluates the potential impact of changes across multiple dimensions—ranging from operational processes and equipment performance to regulatory compliance. This holistic analysis ensures that all facets of the change are thoroughly assessed, helping organizations to understand and mitigate unintended consequences before implementation.
- Control Change Timeframe: AI devises a timeframe for completing change control plan. This enables teams to work accordingly to close change control requests within their specified timeframe.
Achieve up to 70% cost effectiveness
Technology Behind AI based Change Control Recommendation System
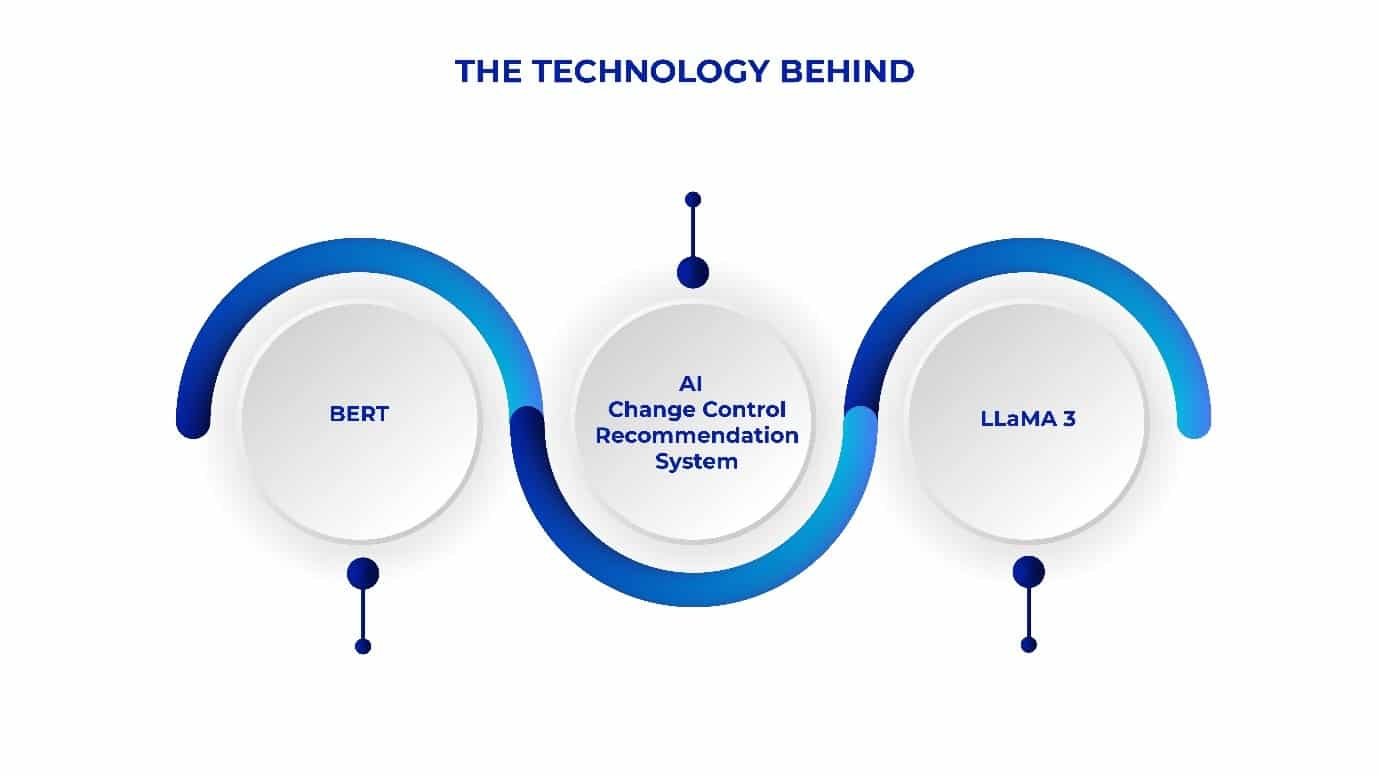
- Exact Matching: Firstly, the system efficiently filters Change Control records by directly matching the Type of Change and the affected area, ensuring that only the most pertinent and relevant cases are selected for review. This reduces the clutter and streamlines the decision-making process.
- Embedding with BERT: Utilizing BERT (Bidirectional Encoder Representations from Transformers), the system converts both the proposed change and existing procedures into vector representations. This method captures the underlying semantics and contextual meanings, enabling the system to identify and compare the most relevant historical cases based on similarity.
- Generation with LLaMA 3: Building on the insights gained from BERT, the system then employs LLaMA 3 to generate detailed implementation plan recommendations. By analyzing similar past change control cases, LLaMA 3 processes the data and provides tailored, context-specific suggestions for the next steps in the change management process.
- Output: Then, structured implementation plan and time estimates for implementing the required plan and change control are produced, offering clear, actionable insights that enhance the decision-making process. These outputs help project teams understand the potential impact and timeline with greater precision, improving overall efficiency.
Delve into the Benefits!
Change control in pharmaceutical industry is undertaken to implement minutest of process and product changes. While digitized solutions promise to reduce change control closing time, there has been no significant acceleration in processes. 70% of change control operations require more time than what is anticipated. Also, redundancy of tasks make the process way more difficult. Implementing AI can really be a great solution in this case. Here are the benefits of using AI based recommendation system in change control in the pharmaceutical industry:
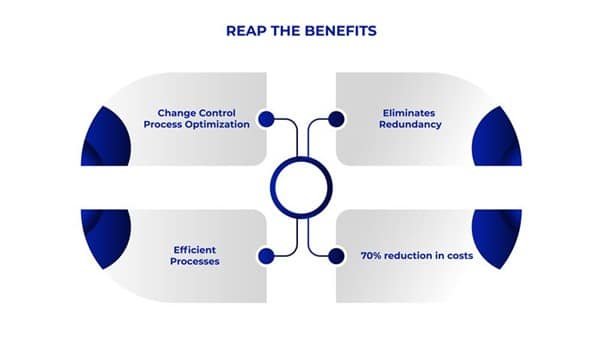
- Change Control Process Optimization: Enables change control process optimization by suggesting change control measures, action plans, impacted departments, etc. This decreases the repeated to-and-fro between departments, HODs and QA. AI recommended timeframe keeps teams on their toes and helps in proper planning for closing change control requests.
- Eliminates Redundancy: Multiple approvals and action plan suggestions from department HOD, QA and CFT makes the process redundant. AI-powered recommendation systems in pharma can eliminate this redundancy by almost 75%, thus enabling change control process optimization.
- Efficient Processes: AI based recommendation system reduces reworking on change control action plans, decreases the approval requests, thereby making processes efficient, the change control implementation faster and enabling change control process optimization.
- Cost Effectiveness: Leverage AI-powered recommendation systems in pharma for Change Control to achieve up to an 70% reduction in costs by automating key processes such as action plan drafting and impacted department identification. This minimizes manual intervention, streamlines operations, and optimizes resource allocation, delivering significant financial savings while enhancing operational efficiency.
Integrating AI into pharma change control systems and processes provides exceptional returns. Traditional systems, while methodical, are often bogged down by inefficiencies, repetitive tasks, and lengthy approval cycles. AI based recommendation system in pharma change control eliminates these hurdles by leveraging historical data, automating workflows, and minimizing manual effort.
By speeding up closure times, reducing redundancies, and optimizing overall efficiency, AI enables pharmaceutical companies to maintain stringent regulatory compliance while enhancing productivity. This innovative approach offers change control process optimization and establishes a new standard for efficiency, agility, and reliability in regulated industries.





























