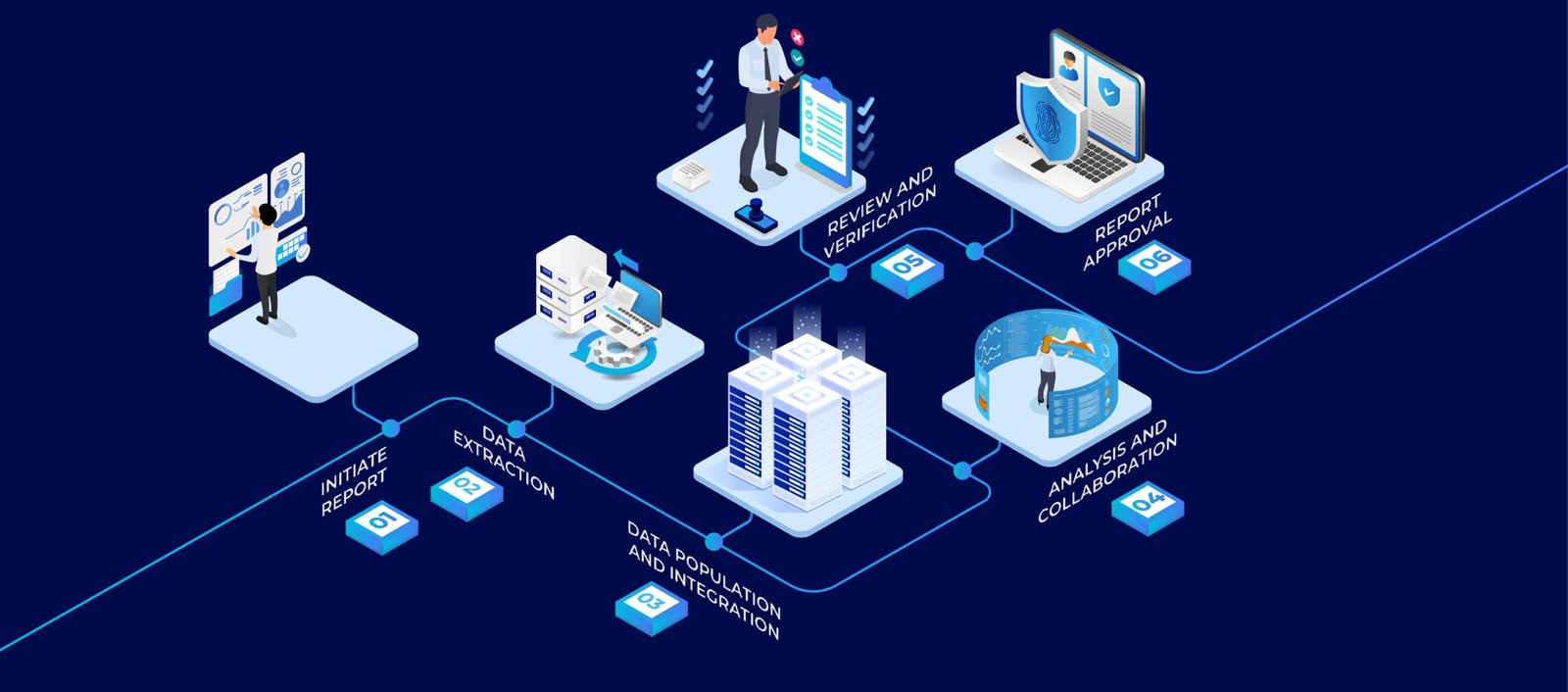
In the dynamic landscape of the Life Sciences industry, ensuring compliance with Good Manufacturing Practices (GMP) is imperative to guarantee the safety, efficacy, and quality of pharmaceutical products. One critical aspect of GMP is the Annual Product Quality Review (APQR), an essential process for evaluating and monitoring the consistency of product quality throughout its lifecycle. APQR is also known as annual product review (APR) in FDA and product quality review (PQR) in EU good manufacturing practice (GMP) guidelines.
It is required to be conducted annually for every product to determine the need for changes in drug product specifications or manufacturing or control procedures. However, challenges in the industry related to Product Quality Reviews (PQR) have highlighted the need for innovative solutions. The article will leave you with a little more understanding about the challenges associated with PQR in the Life Sciences industry and how AmpleLogic, a leading GMP automation technology provider, can elevate the APQR while bridging existing gaps. So, let’s delve into it!
Current Challenges while conducting effective APQR:
- Manual Processes and Data Management: Traditional PQR processes often rely on manual data collection and analysis, leading to inefficiencies, errors, and delays. The sheer volume of data generated in the manufacturing process like Batch data, QC data, Regulatory compliance data, Equipment data, Environmental monitoring data etc. makes it challenging to manage and analyze information accurately.
- Diversity in Templates Across Sites and Product Types: The use of multiple templates across different sites and product types introduces complexities in data standardization and analysis. This heterogeneity hampers the ability to derive meaningful, standardized insights from APQR reports.
- Annual Document Preparation and Delayed Detection: Document preparation occurring annually, and the absence of real-time, actionable insights hinder the industry’s ability to respond promptly to emerging quality issues, potentially affecting product quality and patient safety. The delay in detecting and correcting trends or shifts also results in costly recalls and regulatory actions, posing a significant risk to product quality and compliance.
- Database Maintenance Challenges: Managing a vast database with one document per year for each product becomes a significant challenge. This approach requires extensive storage facility that translates to added real estate cost and can impede efficient retrieval and analysis of historical data because there is a regulatory requirement of maintaining the approved APR documents for a minimum of 11 years.
- Integration Hurdles for Electronic Adoption: The transition to electronic APQR is hindered by integration challenges, making it difficult for companies to seamlessly incorporate electronic processes into their existing workflows.
- Regulatory Compliance: Meeting the stringent regulatory requirements for PQR is a complex task. Frequent changes in regulations and the need for comprehensive documentation add a layer of complexity to ensure compliance with various global regulatory bodies.
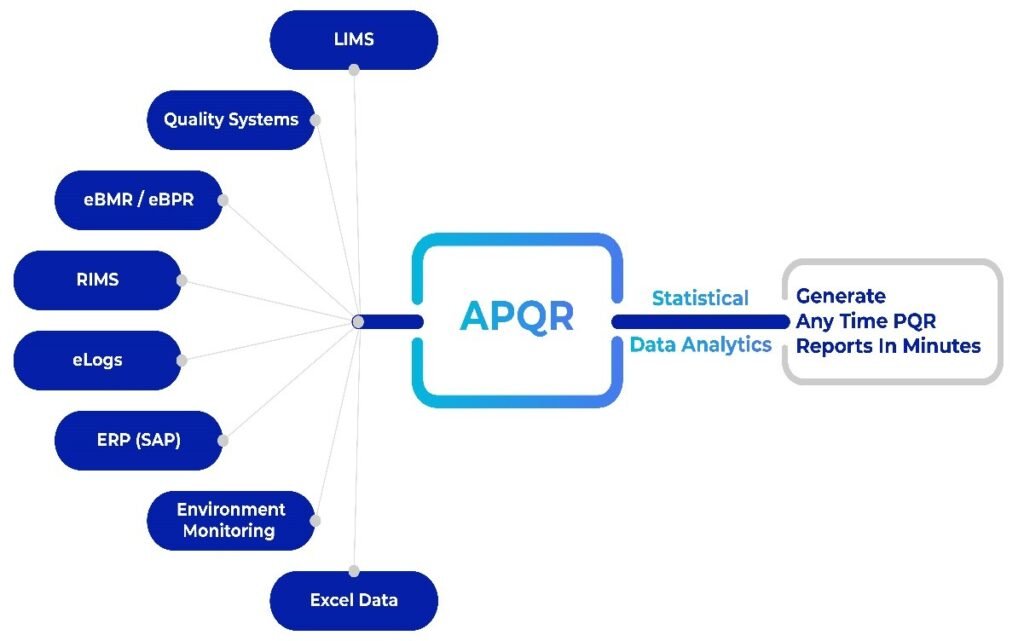
AmpleLogic’s Solution for Elevating APQR
AmpleLogic, a frontrunner in providing innovative solutions for the Life Sciences industry, offers a comprehensive platform designed to address the challenges associated with PQR and elevate APQR processes:
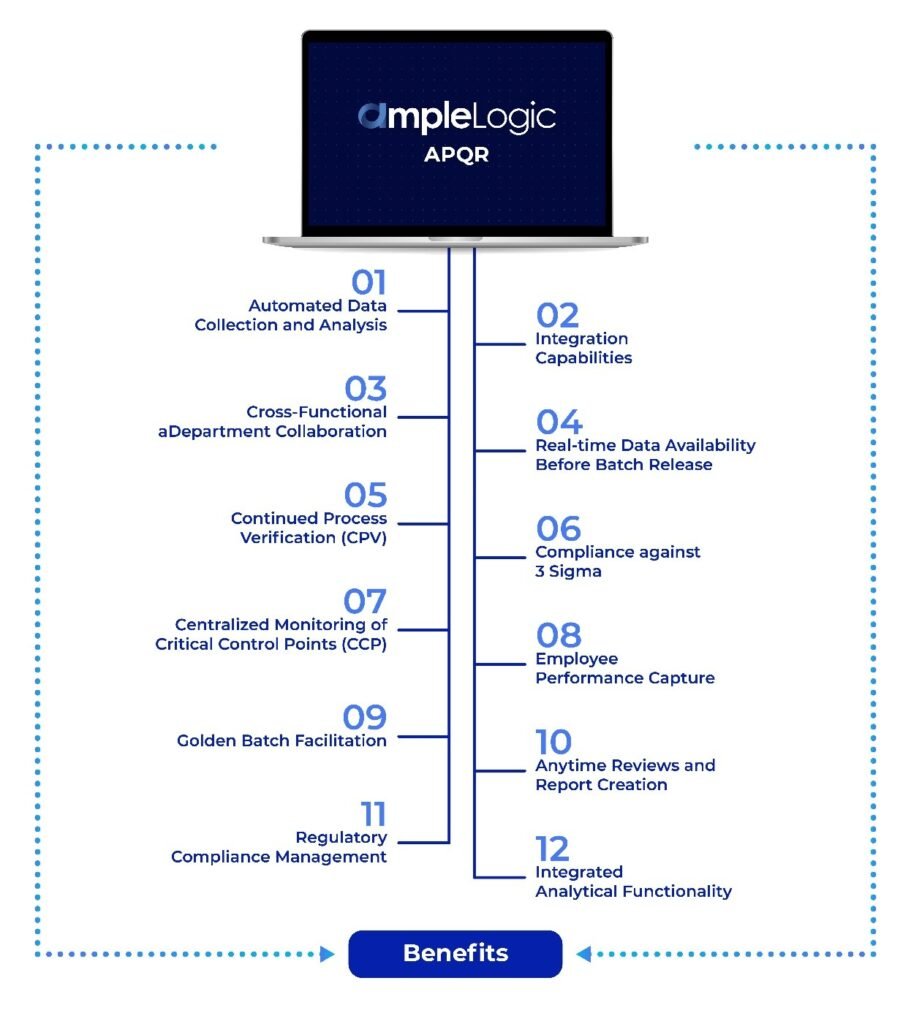
Automated Data Collection and Analysis: AmpleLogic’s platform automates the data collection process, reducing manual errors and streamlining the analysis of vast datasets. This ensures the timely generation of APQR reports, enhancing overall operational efficiency.
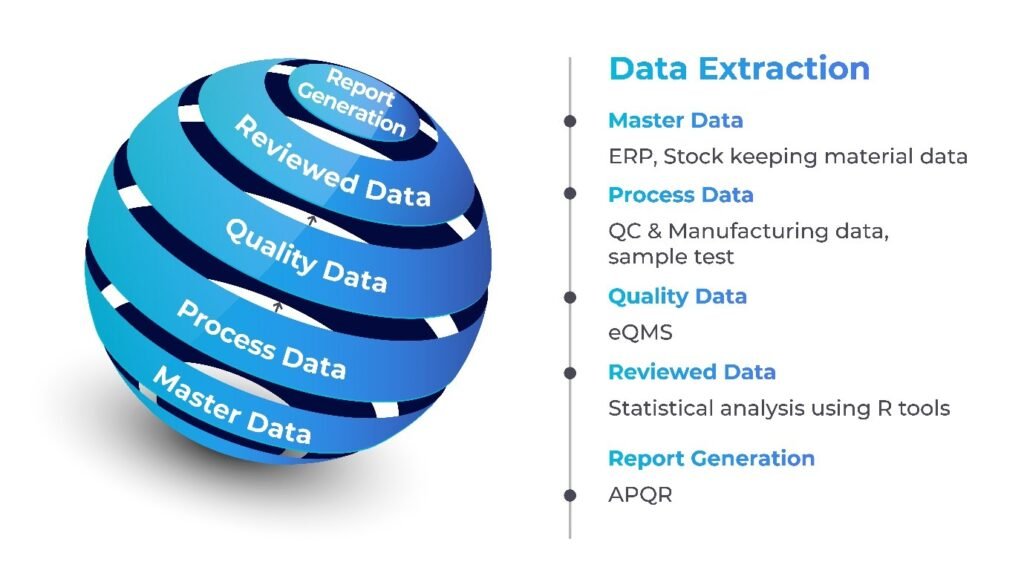
Integration Capabilities: The platform facilitates the integration of diverse data sources, breaking down silos between manufacturing, quality control, and supply chain data. This integration enhances the ability to derive holistic insights, enabling informed decision-making.
Cross-Functional Department Collaboration: Users can see what they’ve been assigned, and various department users can contribute and collaborate on PQR. Users can send all approvals at once, receive email notifications and sign them, add notes or comments, and respond and make changes based on those notes.
Real-time Data Availability Before Batch Release: AmpleLogic’s APQR ensures that all relevant data is available in real-time before batch release, enabling proactive decision-making and minimizing the risk of quality issues escaping detection.
Continued Process Verification (CPV): APQR facilitates CPV by providing continuous monitoring of critical parameters throughout the manufacturing process. This proactive approach ensures ongoing compliance and quality assurance.
- Control charts are used to track critical quality attributes (CQAs) and critical process parameters (CPPs) over time. Any trends, shifts, or out-of-control points on the chart can signal potential issues that need investigation.
- Specification limits are compared to the control chart data to ensure that the process is consistently producing results within the defined specifications.
- Control limits on control charts help identify statistically significant variation in the process. Points beyond these limits may indicate special cause variation that requires investigation.
- Although CPV includes ongoing monitoring of the process, but the initial validation batch data serves as a baseline for expected performance. Deviations from the validated state may trigger further investigation and corrective actions.
- In CPV, Ppk (Process Performance Index) calculations help quantify how well the process is meeting specifications. A high Ppk value indicates a capable process, while a low value may suggest a need for process improvement.
Compliance against 3 Sigma: AmpleLogic’s APQR aligns with statistical process control methodologies, ensuring compliance against 3 Sigma. This data-driven approach enhances the precision and reliability of product quality assessments.
Centralized Monitoring of Critical Control Points (CCP): The APQR system establishes tolerances for Critical Control Points (CCP) and escalates any excursions beyond Proven Acceptable Range (PAR) or outside the Normal Operating Range (NOR). This proactive alerting mechanism ensures swift corrective actions for every batch manufactured.
- Proven Acceptable Range (PAR): PAR is the demonstrated range of process parameters that consistently produces a product meeting its predefined quality attributes. It is determined through the validation process and represents the acceptable variation in the parameters that ensures product quality.
- Normal Operating Range (NOR): NOR is the specified range within which process parameters are expected to operate under routine manufacturing conditions. It is often set within the wider PAR and represents the typical operating conditions for day-to-day production.
Employee Performance Capture: APQR goes beyond product-centric metrics by capturing employee performance data. This holistic approach allows organizations to assess and improve overall operational efficiency.
Golden Batch Facilitation: AmpleLogic’s APQR supports the concept of the “Golden Batch,” allowing organizations to identify and replicate optimal manufacturing conditions consistently.
Anytime Reviews and Report Creation: By simplifying and automating the process, users can create virtual or interim PQRs, allowing them to routinely assess the state of their processes and review the progress of their continuous improvement initiatives.
Regulatory Compliance Management: AmpleLogic’s solution is equipped with built-in compliance features like e-signatures, audit trials as required by 21 CFR Part 11 and EU Annex 11, ensuring that PQR processes adhere to the latest regulatory requirements. This capability enables life sciences companies to stay ahead of regulatory changes and maintain compliance seamlessly.
Integrated Analytical Functionality: AmpleLogic APQR integrates comprehensive analytical functionalities, eliminating the need for external tools like SAS, MiniTab, or SPSS, ensuring a seamless and efficient APQR experience.
In conclusion, addressing PQR challenges in the Life Sciences industry requires innovative solutions to maintain product quality and GMP compliance. By leveraging cutting-edge digital technology for end-to-end automation, drug manufacturers can streamline data collection, analysis, collaboration, and approval processes. AmpleLogic’s platform stands out as a robust solution, offering automated processes, regulatory compliance management, integration capabilities, and real-time monitoring at reduced costs.
Through AmpleLogic’s technology, companies can enhance their APQR processes, bridge existing gaps, and confidently navigate the pharmaceutical industry’s complexities. Modernizing the APQR process also brings benefits such as electronic collaboration, streamlined regulatory report compilation, time savings, and improved data analysis, facilitating quicker and more routine informed decisions regarding product quality.
Modern Approach to Product Quality Review






















