
The pharmaceutical industry relies on critical cleaning processes to ensure the safety of medicines and other pharma products. It is necessary that there is adequate examination of manufacturing equipment and cleaning processes to prevent cross-contamination. Manually evaluating the cleanliness of pharma manufacturing vessels leaves room for inaccuracy. Pharma companies need to re-evaluate their pharma validation process to ensure regulatory compliance set forth by US FDA, EMA and other global agencies.
Traditionally, manual cleaning validation process can be time consuming hindering operational effectivity and product quality. In this article, we will look into how automation of cleaning validation process can promote exceptional results in pharma manufacturing.
Importance of Cleaning Validation
Cleaning Validation is an essential aspect of Good Manufacturing Practices (GMP). The aim of having a detailed cleaning validation process in place is to ensure that no residues from previous product batches contaminate the next batch. Contamination can lead to reduced product efficacy, harmful side effects and even life-threatening reactions in patients. It is hence important that cleaning processes are designed, executed and validated thoroughly to ensure consistent product quality and their effectiveness.
With global regulatory bodies offering guidelines on acceptable levels of contamination, cleaning agents and cleaning processes for different equipment, it is important that pharma companies adhere to these guidelines. Businesses need to submit regular cleaning validation reports to regulatory bodies for compliance checks. In an environment where human error can have serious consequences, the need for precision is paramount.
Challenges of Manual Cleaning Validation
Manual cleaning validation process is time-consuming. Owing to the numerous processes involved like designing of cleaning process, execution of cleaning tasks, sampling and testing for residues, data collection, and report generation; there are higher chances of inaccuracies. Each of these steps is labour-intensive and prone to human error. The complexity of cleaning validation increases with the number of products, types of equipment, and cleaning methods involved.
In manual systems, data entry errors, inconsistencies in documentation, and delays in report generation are common issues. Moreover, manual processes are often siloed, making it difficult to achieve real-time visibility into the cleaning validation lifecycle. These challenges can lead to costly production delays, compliance risks, and potential product recalls. Here are some additional challenges associated with manual pharmaceutical cleaning validation:
- Resource-intensive processes
- Manual calculation and identification of worst-case scenarios
- Challenges with regulatory compliance
- Complexity in data analysis and visualization
- Lack of predictive analysis capabilities
- Manual maintenance of VMP schedules
- Limited audit readiness, not available 24/7
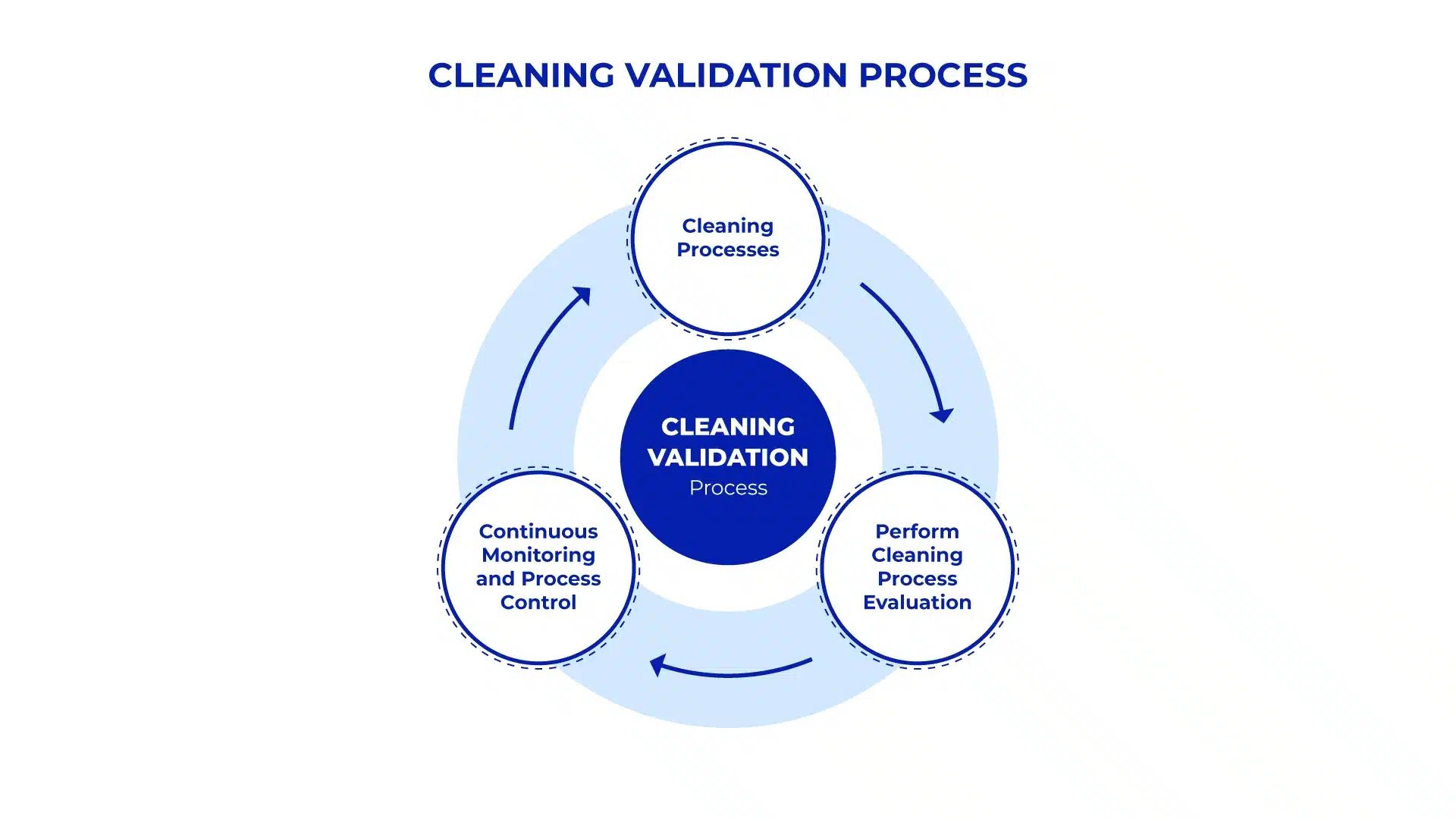
The Role of Automation in Cleaning Validation
Automation of cleaning validation processes addresses several challenges by integrating and streamlining the entire workflow. Automated systems leverage advanced software to manage master data, perform calculations, generate reports, and monitor processes in real time. Here’s how automation is transforming cleaning validation:
- Designing Cleaning Processes: Automated systems allow users to design and store cleaning processes within a centralized database. This includes all relevant data, such as product details, equipment specifications, cleaning agents, and critical process parameters (CPP) for cleaning procedures. The system ensures that these processes are compliant with regulatory guidelines, reducing the risk of non-compliance.
- Performing Cleaning Process Evaluations: Automation enhances the evaluation phase by generating reports and calculations with a high degree of accuracy. For example, the system can automatically calculate the Maximum Allowable Carry Over (MACO) and generate Worst Case Reports, which are essential for demonstrating compliance. These reports are created using predefined templates that align with regulatory requirements, ensuring consistency and accuracy.
- Continuous Monitoring and Process Control: Automated cleaning validation systems include features like process schedulers, which help streamline cleaning cycles and ensure that they are completed on time. These systems also offer real-time monitoring capabilities, providing immediate alerts in case of deviations from the standard process. When a deviation occurs, the system requires the user to input the reason, which is then reflected in the efficiency reports. This level of control and visibility reduces the likelihood of errors and enhances overall process efficiency.
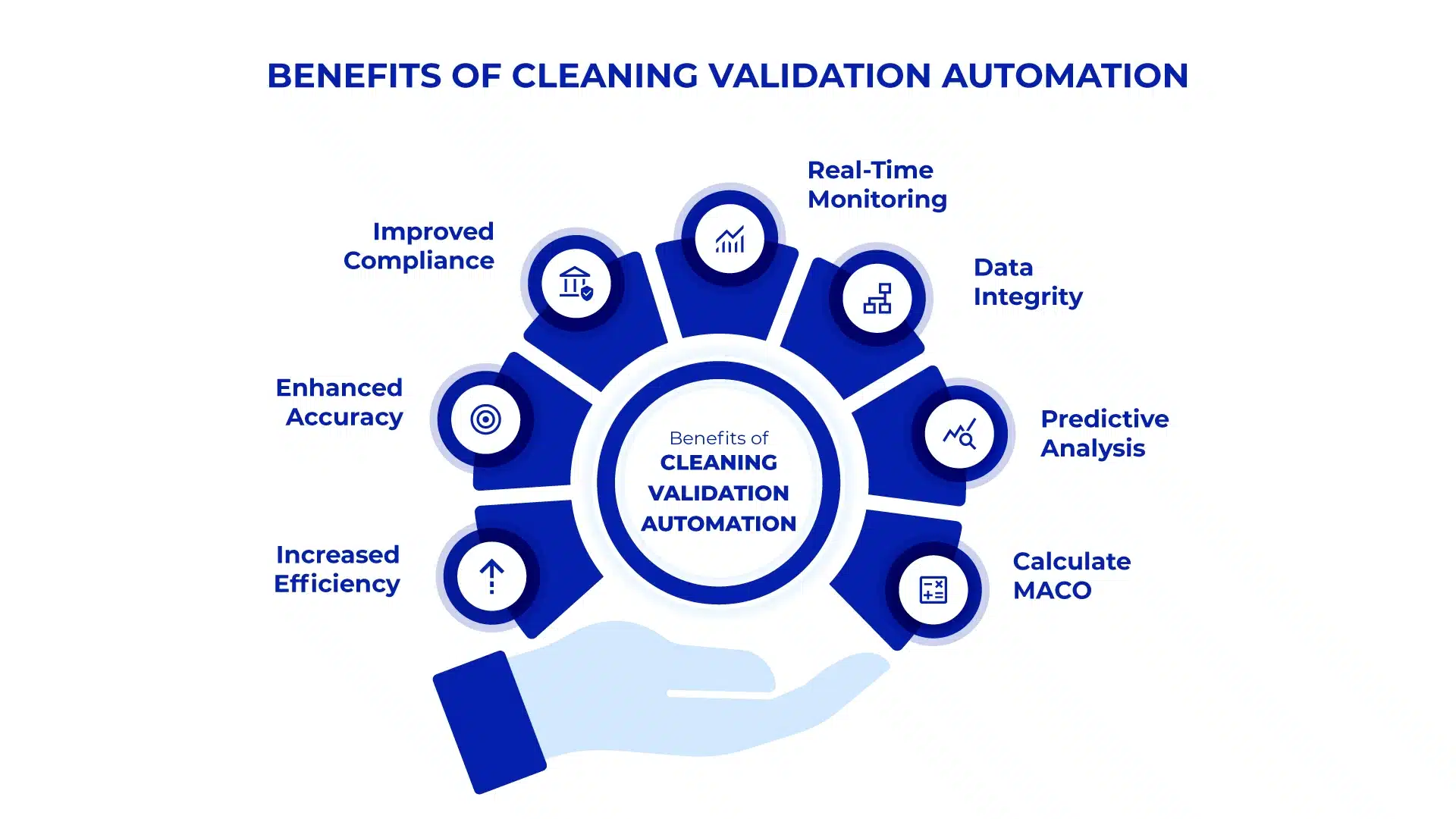
Benefits of Automating Cleaning Validation
The automation of cleaning validation processes offers numerous benefits:
- Increased Efficiency: Automation reduces the time and labour required to complete cleaning validation tasks, allowing companies to focus resources on other critical areas.
- Enhanced Accuracy: Automated systems minimize human error, leading to more accurate data collection, calculations, and reporting.
- Improved Compliance: By adhering to regulatory guidelines through automated processes, companies can reduce the risk of non-compliance and associated penalties.
- Real-Time Monitoring: Automation provides real-time visibility into cleaning processes, enabling proactive management and quicker response to any issues that arise.
- Data Integrity: With all data stored and managed in a centralized system, the integrity of information is maintained, making audits and inspections more straightforward and less stressful.
- Predictive Analysis: Use advanced algorithms to forecast risks, optimize processes, and ensure regulatory compliance with real-time decision-making and prevention of contamination.
AmpleLogic’s Cleaning Validation Software Leading the Way!
AmpleLogic’s Cleaning Validation software is an excellent solution to automate your cleaning validation processes. The solution is equipped with some unique features that makes it ideal for the pharma industry. With excellent integration with equipment and other systems such as MES, LIMS, DMS, APQR, etc; you can seamlessly collect data and monitor cleaning processes. Get real-time alerts for deviations, calculate Maximum Allowable Carryover (MACO), schedule cleaning processes and indulge in continuous validation of cleaning processes. AmpleLogic Cleaning Validation software helps you in improvement of processes, enhancing product quality and adhering with compliances.

The Future of Cleaning Validation
As the pharmaceutical and life sciences industries continue to evolve, the role of automation in cleaning validation will only become more critical. Future advancements may include the integration of AI and ML to further enhance the capabilities of automated systems. For instance, AI could be used to predict potential deviations before they occur, enabling even greater levels of process control.
The automation of cleaning validation processes marks a significant step forward for the pharmaceutical and life sciences industries. By embracing automation, companies can achieve greater efficiency, accuracy, and compliance, ultimately leading to safer and more reliable products. As technology continues to advance, the potential for further improvements in cleaning validation is vast, promising a future where manual errors and compliance risks are minimized, and the focus can remain on innovation and patient safety.





















