
The pharmaceutical industry has been expanding steadily across developed and developing markets. With growing regulatory pressure and the shift to digital systems, pharmaceutical companies are now being pushed to re-evaluate their existing documentation practices. One of the major roadblocks remains the shift from traditional manual logbooks to digital logbooks.
Most pharmaceutical formulation and chemical manufacturing sites still rely on paper-based instruments and equipment usage logbooks. Depending on the organization’s size, they maintain between 500 to 2000 logbooks across various product categories.
Challenges with Manual Logbooks in the Pharmaceutical Industry
Tracking, updating, and maintaining versions of these manual logbooks is repetitive and error-prone. The risk of duplication is high, and such lapses can raise regulatory concerns. Below are some of the key challenges organizations face:
- Compliance and regulatory gaps:
Manual entries increase the risk of human error. Missing entries, overwritten data, or inconsistent formats can result in non-compliance during audits. - Productivity and operational delays:
Paper logs take time to fill, review, and retrieve. This slows down processes like batch release, investigations, and shift handovers. - Data inconsistency:
Logbooks maintained by different personnel often follow different formats. This leads to fragmented records and makes any form of analysis difficult. - Inventory tracking:
For pharmaceutical manufacturers, keeping real-time tabs on material movements and cleaning schedules is critical. Manual logs don’t support this need. - Risk management:
Without centralized records, identifying deviations or anomalies becomes reactive. Gaps in documentation directly affect risk mitigation.
Types of Logbooks Maintained Across Facilities
Manufacturing areas, engineering teams, plant maintenance, and warehouses all maintain separate sets of logbooks. Even when the process is standard, each site or department may use a slightly different format.
Some commonly maintained logbooks include:
Area Cleaning Record
- Preparation and Usage of Disinfectant Solution Record
- Washing Area Cleaning Record
- Calibration Test Record
- Area Sequential Log
- Equipment/Instrument Sequential Log
Due to non-standard printed log formats, the same log type may exist in multiple variants across departments or sites.
According to operational data from pharma facilities, out of 1000+ logbooks in circulation, about 60% are used daily, while the rest follow weekly, monthly, quarterly, or half-yearly usage patterns.
Digitalization of Logbooks
Digitalization replaces manual logbooks with a standardized electronic format. The goal is not just to switch from paper to screen but to harmonize the logs, eliminate unnecessary duplicates, unify formats, and define clear data fields.
This shift is only effective when the business and its technology partner jointly define the scope and structure of digital logs.
Benefits of Digital Logbooks
- Real-time visibility: Operations teams can track updates without waiting for physical logbooks to be reviewed.
- Fewer errors: Predefined fields, dropdowns, and validations reduce manual input errors.
- Centralized data: Records are easier to retrieve during audits or internal investigations.
- Reduced overhead: No need for printing, storing, or manually archiving logbooks.
- Better coordination: Multiple departments can access the same log in real time, removing delays caused by physical handovers.
Modern eLogbook systems also come with offline functionality, especially for restricted-access areas like aseptic processing zones where network connectivity is limited.
The Road Ahead
At first glance, the scale of the problem seems large, hundreds or even thousands of logs per site. But in practice, around 80 harmonized digital logbooks can handle about 90% of daily and weekly requirements.
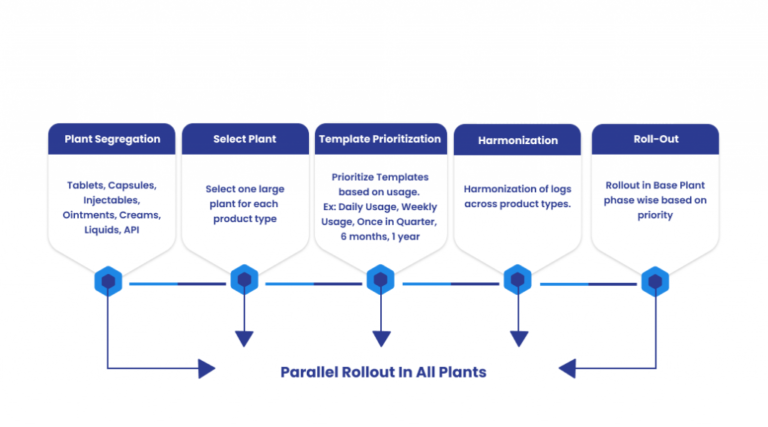
Organizations operating across multiple sites and with varied product portfolios can follow a phased approach:
- Identify logs used most frequently
- Eliminate duplicates and standardize formats
- Roll out digital logs with basic user training
- Integrate offline modules for classified areas
This phased method helps large teams implement change without disrupting operations.
Final Note
The longer companies stay with manual logbooks, the harder it becomes to scale, comply, and respond quickly to audits or deviations. Paper-based systems were built for a different era. For companies running multiple shifts, products, and regulatory regimes, harmonized eLogbooks offer cleaner records and fewer surprises.
Switch from paper trails and shared folders to an electronic Logbook System (eLogs). Schedule a demo with our team to see how easy the transition can be. For more information, visit Amplelogic Resources.

















