
If you have ever felt overwhelmed by the mountain of paperwork and regulations in the life sciences industry, well, you are not alone. The life sciences industry operates under stringent regulatory oversight, where documentation accuracy and compliance are not just operational necessities but legal imperatives. It is always about ensuring your organization is forever ready for an audit, never misses a regulatory update, and can prove, at a moment’s notice, that every process is under control. This is where an efficient compliance document management system becomes your secret weapon.
AmpleLogic’s document management system exemplifies this by integrating AI along with advanced features such as electronic signatures, version control, and automated workflows to meet global standards while streamlining operations. This article examines the crucial role of these systems in ensuring compliance, mitigating risks, and driving innovation in the life sciences.
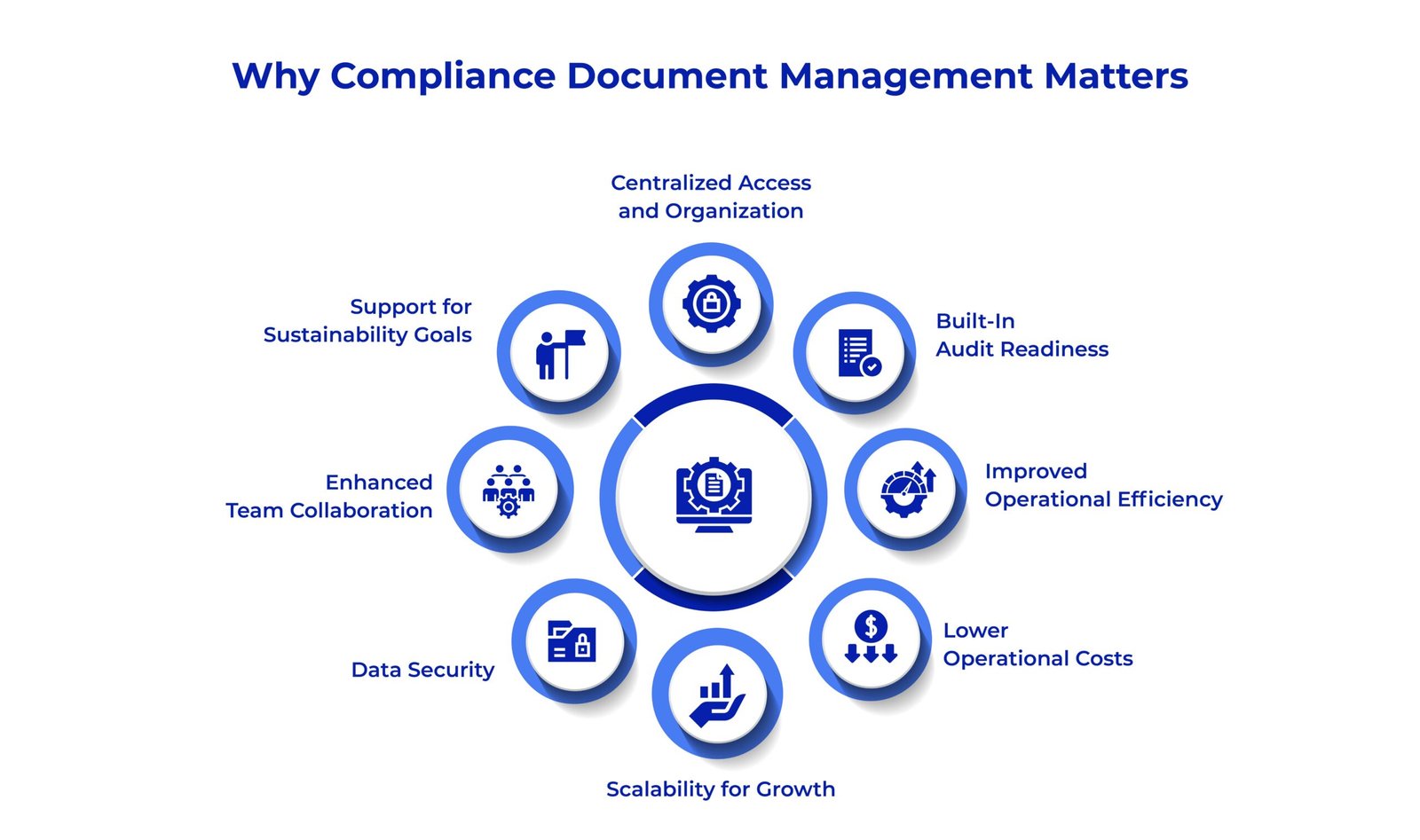
Why Compliance Document Management Matters in Life Sciences?
Imagine you are preparing for an FDA inspection. Every document, from clinical trial records to SOPs, must be accurate, up-to-date, and instantly accessible. A single missing or outdated file could mean delays, fines, or worse. That’s why a compliance document management system isn’t just a nice-to-have; it’s essential for life sciences organizations. The benefits of Compliance Document Management in lifesciences are –
- Centralized Access and Organization
All compliance-related documents are stored in a single, secure repository which are structured, searchable, and version-controlled.
- Built-In Audit Readiness
With real-time tracking, version histories, and complete audit trails, your team can respond to inspections without last-minute scrambling.
- Improved Operational Efficiency
Automated workflows for document approvals, reviews, and updates reduce manual workload, accelerate processes, and improve consistency.
- Lower Operational Costs
Reduced reliance on paper, minimized physical storage, and streamlined compliance workflows all contribute to measurable cost savings.
- Scalability for Growth
As your organization expands in size, locations, or regulatory obligations, the system scales effortlessly to meet new demands.
- Data Security
Advanced access controls, encryption, and permission settings protect sensitive content and prevent unauthorized access.
- Enhanced Team Collaboration
A centralized platform allows cross-functional teams to collaborate seamlessly on documents, with real-time updates and comment tracking.
- Support for Sustainability Goals
Digital-first document handling reduces paper usage, energy consumption, and supports environmental compliance initiatives.
Understanding GxP Document Management in Life Sciences
The Pillars of GxP Compliance
GxP encompasses a suite of quality guidelines, including Good Manufacturing Practice (GMP), Good Laboratory Practice (GLP), and Good Clinical Practice (GCP), that ensure products are consistently produced and controlled according to quality standards. GxP document management is at the core of regulated industries. It’s all about Good Practices, whether in manufacturing, clinical trials, or laboratory work. The right system ensures:
- Every document is stored securely and can be accessed only by authorized personnel.
- Version control is automatic; you’ll never have to worry about someone using an outdated SOP.
- Audit trails are built in, so you always know who did what and when.
AmpleLogic’s DMS platform addresses these requirements through configurable workflows that align with GMP and GCP processes, reducing the risk of human error during manual document handling.
Challenges in Manual GxP Compliance
Organizations relying on paper-based systems or disjointed digital tools face significant risks:
- Version Discrepancies: Uncontrolled drafts circulating among teams can lead to deviations during audits.
- Delayed Approvals: Manual routing of documents for signatures prolongs cycle times, impacting time-to-market.
- Data Integrity Gaps: Lack of audit trails for corrections or annotations raises red flags during FDA inspections.
By transitioning to a 21 CFR Part 11 compliant document management system, life sciences firms can automate approval workflows, enforce electronic signatures, and maintain immutable records, key pillars of GxP adherence.
Also Read – Top 9 Challenges and Solutions for eDMS Software in Pharma Industry
FDA 21 CFR Part 11 Compliance: Navigating Electronic Records and Signatures
The FDA’s regulation mandates strict controls for electronic records and signatures to ensure they are trustworthy, reliable, and equivalent to paper records. A 21 CFR Part 11 compliant document management system must include:
- Electronic Signatures: Linking signatures to unique user credentials.
- Audit Trails with Immutable Timestamps: Recording the date, time, and user identity for every action, including document edits, approvals, or deletions.
- Access Controls: Restricting document access based on roles to prevent unauthorized modifications.
AmpleLogic’s document management system integrates signing and timestamping to meet these requirements, ensuring data integrity even during multi-year clinical trials.
SOP Document Management: Standardizing Processes Across the Organization
Standard Operating Procedures (SOPs) define the exact methods for critical tasks, from equipment calibration to adverse event reporting. Effective SOP document management ensures:
- Uniform Training: Distributing the latest SOP versions to all personnel, reducing deviations caused by outdated practices.
- Cross-Functional Collaboration: Enabling R&D, manufacturing, and quality teams to comment on drafts within a unified platform.
- Periodic Review Automation: Scheduling SOP reassessments at predefined intervals to maintain compliance with evolving regulations.
AmpleLogic streamlines SOP lifecycle management through AI-driven analytics that identify redundancies or conflicts between procedures, ensuring alignment with ICH (International Council for Harmonisation) guidelines.
Features of a Modern Compliance Document Management System
The features of the latest modern compliance document management system are –
- Electronic Signatures
For internal approvals requiring user credentials, ampleLogic’s FDA document compliance module supports electronic signatures.
- Automated Audit Trails and Reporting
Regulatory agencies increasingly demand granular audit trails that capture:
- User login/logout times.
- Document access attempts (successful or failed).
- Field-level changes in electronic forms.
AmpleLogic’s DMS generates preconfigured reports for FDA inspections, reducing preparation time from days to minutes.
- Integration with Quality Management Systems (QMS)
A seamless connection between document control and QMS modules enables:
- Deviation Management: Linking SOP revisions to CAPA (Corrective and Preventive Action) plans.
- Change Control: Automatically updating affected documents when regulatory guidelines change.
- Training Management: Assigning revised SOPs to personnel based on their roles.
- Integration of LMS as well as eBMR
- DMS helps by recording details to ensure Correct labeling, batch codes, and packing materials used, often included as annexures to BMRs.
- It also helps in real-time data, such as weight variation, hardness, and in-process records, to maintain batch consistency during production.
- Linked Change control ensures that any changes in the manufacturing process are reflected in BMRs via proper documentation in DMS
- DMS also ensures BMRs are created, reviewed, and approved according to GxP and 21 CFR Part 11, tracks audit trials, electronic signatures, and version control.
Why Choose AmpleLogic?
- All-in-One Platform: Manage every type of compliance document in a single, user-friendly system.
- Seamless Integration: Work with familiar tools like Microsoft Office, making adoption easy for your team.
- Continuous Validation: Stay up-to-date with evolving regulations, with ongoing validation and support included.
- Scalable and Secure: Whether you’re a startup or a global enterprise, AmpleLogic grows with you, keeping your data safe at every step.
Best Practices for Compliance Document Management
To get the most out of your compliance document management system, keep these tips in mind:
- Standardize Procedures: Use templates and clear guidelines for all documents.
- Automate Where Possible: Let the system handle routine tasks, focus your energy where it matters.
- Regular Audits: Use built-in audit trails to review processes and spot gaps before regulators do.
- Train Your Team: Ensure everyone knows how to use the system and understands the importance of compliance.
Also Read – Streamlining Pharmaceutical Manufacturing with DMS Software
The Future of Compliance Management: AI and Predictive Analytics
Emerging technologies are transforming regulatory document management systems from reactive tools to proactive platforms. AmpleLogic’s document management system, which is powered by AI, for example, analyzes historical audit data to predict compliance risks, such as:
- Expiring Documents: Flagging SOPs approaching their review dates.
- Training Gaps: Identifying employees who haven’t acknowledged updated procedures.
- Inconsistencies: Detecting conflicts between internal SOPs and newly published FDA guidance.
By addressing these issues preemptively, organizations can avoid costly regulatory actions and maintain uninterrupted operations.
The Bottom Line
In this future-driven, highly regulated world of life sciences, a modern compliance document management system is your ticket to smoother operations, fewer headaches, and total audit confidence. Whether you’re focused on GxP document management, an efficient regulatory document management system, streamlined SOP document management, bulletproof FDA document compliance, or a 21 CFR Part 11 compliant document management system, AmpleLogic delivers the tools and support you need to stay ahead.
Explore how AmpleLogic can help your organization stay audit-ready, risk-resilient, and digitally advanced. If you are interested in more such articles, find us here – AmpleLogic’s Resource!





















