Digitalizing Laboratory Planning and Scheduling: Best Practices and Insights

Laboratory operations are experiencing a growing demand to produce faster results while making the most of limited resources. In pharmaceutical and testing environments, quality and compliance are essential. To stand out, effective scheduling and planning in QC Labs are crucial. This article examines practical approaches to digitalizing laboratory planning and scheduling based on industry implementations and documented results.
The Complexity of Laboratory Scheduling
Quality Control laboratories manage scheduling challenges that significantly exceed those in manufacturing environments. A comparative analysis reveals that while a typical manufacturing process might coordinate 100 batches throughout production, QC labs must orchestrate approximately 1,000 different tests and tasks- a tenfold increase in complexity. This disparity stems from several operational realities:
- Competing priorities across raw material testing, in-process controls, intermediate tests, final API tests, and stability studies
- Diverse analyst qualification profiles requiring precise assignment matching
- Unpredictable incoming sample volumes and timing
- Being downstream in the supply chain clearly puts pressure on delivery.
- Variable test durations and resource requirements
Current scheduling and planning in QC Labs approaches, which typically rely on whiteboards, spreadsheets, or basic laboratory information management system (LIMS) functionality, struggle to address these complexities. Industry analysis indicates that – “Most labs today use MS Excel-based tools, whiteboards, and basic LIMS to define assignments, yet these remain primarily manual scheduling techniques that consume 2-3 hours daily from each supervisor.“
The Case for Digital Laboratory Planning and Scheduling
Implementation data from pharmaceutical and laboratory environments demonstrates specific performance improvements through digital scheduling and planning in QC Labs solutions. The benefits include:
- 20% reduction in test and raw material release times
- 15% decrease in drug safety stock and raw material inventory requirements
- 80% reduction in scheduled labor through optimized resource allocation
- Improved adherence to test prioritization protocols
- Reduced risk of quality deviations through more timely testing
These metrics represent significant operational and financial advantages, particularly as the industry faces $112 billion worth of 15 top-brand drugs coming off patent in the next five years, intensifying cost pressures between 2023 and 2029.
Best Practices and Implementation Approaches
Digital quality control lab scheduling brings structure and visibility to complex testing workflows. To make the most of it, here are key practices that help labs operate with better coordination, clearer priorities, and consistent delivery timelines.
1. Digital Twin Modeling
Digital twins are virtual replicas of your lab setup, mirroring instruments, staff schedules, and sample flow. By simulating test scenarios, planners can predict bottlenecks, optimize resource allocation, and identify the most efficient workflows. This approach allows testing of scheduling and planning in QC Labs scenarios without operational disruption, providing actionable insights for optimization.
Digital twin implementations enable laboratories to:
- Visualize scheduling decision impacts before execution
- Identify resource constraints proactively
- Test process improvements in simulated environments
- Optimize resource allocation across multiple scenarios
Example: A digital twin can simulate the impact of doubling batch sizes during stability testing, highlighting where capacity expansions or shifts in staffing are needed.
2. Critical Path Prioritization
Successful quality control lab scheduling implementations prioritize critical path testing, ensuring that tests with the longest duration (in analyst time and instrument time) begin before shorter tests.
Industry implementation data confirms: “When samples from different products arrive at the lab and these samples, once campaigned, have a long test in terms of analyst hands-on time and instrument time, overall schedule adherence improves by starting these long tests first.“
Time-sensitive tests (e.g., in stability or QC release) should be scheduled first. Digital systems can automatically identify these based on regulatory timelines, batch release deadlines, or R&D milestones, preventing delays in production or clinical trials. Implementing the tagging feature that flags high-priority samples within your LIMS or eQMS might help you further.
3. Strategic Test Campaigning
Effective digital scheduling and planning in QC Labs maximizes test campaigning (grouping similar tests) while maintaining service levels. This approach:
- Minimizes setup and changeover time
- Increases analyst efficiency through standardized workflows
- Optimizes instrument utilization rates
- Balances efficiency with delivery requirements
Counterintuitively, during backlog situations, increasing campaign size often proves more effective than expediting individual samples. In one documented implementation, it states: “Both supply chain and QC laboratories should aim to expand campaign size, understanding that this may lead to minor delays for certain samples while enhancing overall efficiency and enabling the lab to regain capacity.”
Grouping similar tests or samples minimizes changeovers and equipment downtime. For example, grouping HPLC tests using the same method reduces calibration overhead and boosts throughput. Modern scheduling systems can evaluate multiple campaigning scenarios and select the approach that best balances efficiency gains with service level requirements.
4. Resource Planning Integration
Resource planning provides the foundation for effective scheduling. Before implementing detailed quality control lab scheduling, laboratories must establish accurate resource requirements based on forecasted demand.
Integrated digital solutions connect resource planning with scheduling, enabling laboratories to:
- Project staffing needs based on validated demand models
- Identify short-term resource gaps that require strategic capacity adjustments
- Develop strategies for identifying long-term needs related to hiring or outsourcing.
- Allocate resources optimally across competing priorities
The relationship between planning and scheduling operates hierarchically but interdependently, with resource planning at the strategic level and scheduling at the tactical, day-to-day level. Resource planning tools must account for the full complexity of laboratory operations, including analyst qualifications, instrument capabilities, and test requirements.
5. Real-Time Adaptation Capabilities
The most valuable aspect of digital quality control lab scheduling solutions is their ability to adapt to changing conditions. As noted by implementation specialists, “Scheduling is fundamentally a rescheduling problem. No matter how good your current schedule is, real-world anomalies will invalidate it.“
Advanced scheduling systems provide:
- Automated responses to input data changes
- Rapid adaptation to manual inputs like analyst absences
- Optimized schedule recalculation with minimal disruption
- Efficient communication of changes to affected personnel
This “sense and respond” capability proves particularly valuable in dynamic laboratory environments where priorities shift rapidly. When an analyst calls in sick or an urgent test arrives, the system recalculates the optimal schedule and distributes updated assignments automatically.
The real-time adaptation capability addresses one of the most significant limitations of manual scheduling approaches: the inability to quickly respond to changes without creating cascading disruptions. Digital systems can evaluate the impact of changes across the entire schedule and make targeted adjustments that minimize overall disruption.
6. Integration with Other Digital Initiatives
Amplelogic LIMS integrates AI with QMS and QC planning to track trends and potential deviations across batches by analyzing historical test results, raw material data, and batch records. If a deviation occurred in a previous batch, the system automatically flags similar risks in the current batch. This helps QC lab scheduling planners shift priorities, whether that means assigning senior analysts, adding verification steps, or adjusting timelines, so quality risks are caught early and managed effectively.
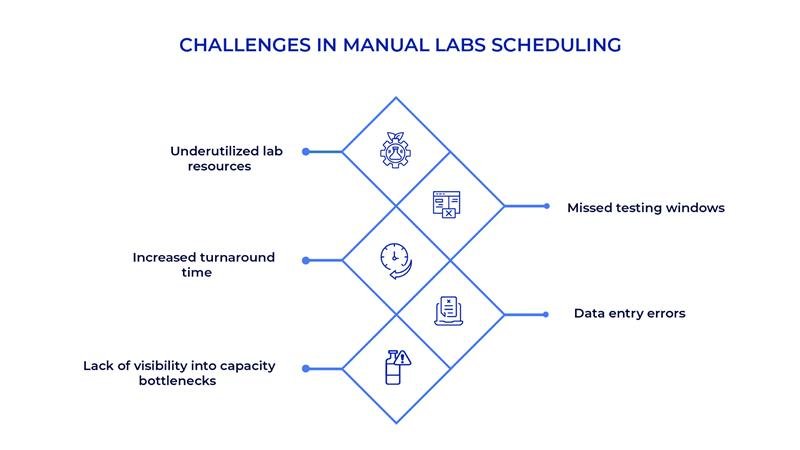
Implementation Requirements for Digital Scheduling
Successful digital scheduling and planning in QC Labs implementations address several critical factors:
1. Data Integration Architecture
Smooth integration of LIMS with scheduling systems eliminates the need for duplicate data entry. Implementation experience shows that simple integration allowing samples and tests to flow automatically into scheduling systems maintains data integrity and reduces administrative overhead.
The integration framework must manage both the initial system connection and continuous data alignment between platforms in QC lab scheduling. Changes in test requirements, analyst qualifications, or instrument availability must propagate automatically between systems to maintain scheduling accuracy. The integration of Quality Management System with the batch release checklist of APQR also helps in streamlining the deviations, further enabling QC planning to be compliant with the batch checklist.
2. Scheduling Parameter Configuration
Quality control lab scheduling platforms require configuration to incorporate all critical operational parameters:
- Analyst qualifications and proficiency levels
- Test due dates and priority classifications
- Critical path identification algorithms
- Workload balancing parameters
- Campaign optimization rules
- Shift patterns and availability constraints
These criteria need to be consistent with the goals of the laboratory and the needs of the business. If on-time delivery is the primary goal, the system should prioritize due dates over efficiency. If cost reduction dominates, campaign optimization might take precedence.
The configuration process requires a deep understanding of laboratory operations and system capabilities during QC lab scheduling. Organizations should involve experienced laboratory managers in the process to ensure the system reflects actual operational requirements and constraints.
3. Change Management Strategy
Implementation requires effective change management. Laboratory staff often resist transitioning from familiar manual processes to digital systems. Successful implementations focus on demonstrating tangible benefits and providing comprehensive training.
The transition should proceed in phases, with clear communication about how the new system addresses existing pain points. Involving key stakeholders in configuration and testing builds buy-in and ensures the solution meets operational requirements.
4. Performance Measurement Framework
Clear performance metrics must be established to measure the impact of digital scheduling and planning in QC Labs:
- Cycle time reduction
- On-time delivery improvement
- Resource utilization rates
- Labor efficiency gains
- Cost reduction
These metrics should be monitored through dashboards providing visibility into laboratory performance. Implementation data confirms: “Just as athletes respond to scoreboards showing game results, production and QC Lab scheduling and planning personnel respond to key metrics visibility.”
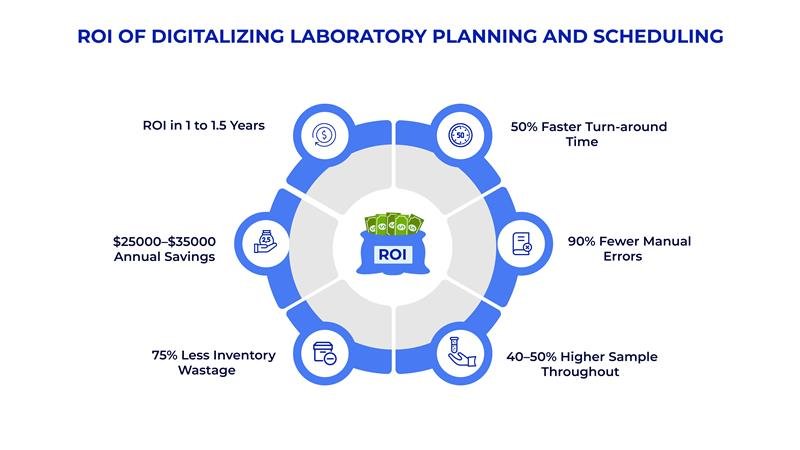
Emerging Trends in Laboratory and Schedule Planning
Several developments are reshaping laboratory scheduling approaches:
- AI and Machine Learning Applications: Advanced scheduling systems now incorporate AI and machine learning to predict bottlenecks, optimize resource allocation, and continuously improve scheduling algorithms based on historical performance data.
- IIoT and Automation Integration: Integration of quality control lab scheduling systems with Industrial Internet of Things (IIoT) devices and laboratory automation equipment enables real-time tracking of sample processing and automatic schedule updates based on actual progress rather than estimates.
- Cloud-Based Deployment Models: Cloud-based scheduling solutions offer increased flexibility, accessibility, and scalability, allowing laboratories to implement sophisticated scheduling capabilities without significant infrastructure investments.
Conclusion
Digitalizing laboratory planning and scheduling represents a documented opportunity to improve efficiency, reduce costs, and enhance service levels. By implementing digital twin modeling, prioritizing critical path testing, optimizing test campaign management, integrating with resource planning, and enabling real-time adaptation, laboratories can transform their operations to meet increasing demands.
As market pressures intensify, with $112 billion worth of name-brand drugs set to come off patent in the next five years, laboratories that implement digital scheduling solutions position themselves advantageously. The investment addresses immediate operational needs while building the foundation for continuous improvement and sustainable competitive advantage.
These strategies, based on industry experience, help laboratory leaders effectively navigate scheduling and planning in QC Labs challenges, and Amplelogic can help you with its QC lab planning software and more.





















