Compliance is a critical operational strategy in high-stakes manufacturing. As regulatory requirements continuously evolve and documentation must be impeccable, manufacturers face mounting pressures to stay aligned with industry standards while managing risk. The complexity of manually handling compliance—tracking regulatory changes, maintaining accurate batch records, and ensuring audit preparedness—creates significant challenges, leaving room for costly errors and inefficiencies.
RIMS (Regulatory Information Management System) and eBMR (Electronic Batch Manufacturing Records) address compliance challenges precisely. These advanced solutions automate and streamline the compliance process, enabling manufacturers to stay ahead of evolving regulations. They ensure real-time compliance tracking, centralized data management, and always-auditable batch records, significantly reducing risks tied to manual oversight.
Why Automation is Critical for Manufacturing Compliance
Manufacturers today are battling a landscape of constant regulatory changes, growing documentation requirements, and ever-increasing audit scrutiny. Manual compliance management methods—relying on spreadsheets, paper-based records, and disjointed systems—often lead to inconsistencies, missed deadlines, and compliance failures. The human error factor is high, as data entry mistakes and oversight in tracking can trigger expensive fines or, worse, damage a company’s reputation.
This is why automation has become a necessity in the industry. Automation helps manufacturing companies meet compliance requirements more efficiently by reducing errors, streamlining documentation, and providing real-time regulatory updates. With a robust Compliance Management System, manufacturers can eliminate bottlenecks, ensure audit readiness, and ensure continuous adherence to ever-evolving regulations.
Understanding RIMS: The Backbone of Compliance Management
The RIMS (Regulatory Information Management System) is a game-changing solution for automating and streamlining compliance management. As the backbone of the compliance process, RIMS centralizes all regulatory information, ensuring that manufacturers always remain audit-ready. Here are the standout features of RIMS:
Centralized Data Management: RIMS ensures that all compliance-related data is stored in a single, secure location, making tracking, auditing, and updating far simpler.
Automated Reporting: With RIMS, manufacturers can generate compliance reports aligned with the latest regulatory standards, whether they are FDA guidelines or GMP regulations.
Real-Time Compliance Updates: RIMS is constantly updated with new regulatory changes, ensuring manufacturers stay ahead of the curve without manual intervention.
RIMS also addresses common compliance pain points, such as fragmented systems, missed deadlines, and inadequate audit preparation. Manufacturers can focus on their core business operations by automating compliance tasks while maintaining full regulatory compliance.
AmpleLogic’s years of expertise in regulatory compliance and technology have culminated in developing RIMS—a platform designed to alleviate manufacturers’ unique challenges regarding compliance.
eBMR: Streamlining Batch Manufacturing for Compliance Excellence
Electronic Batch Manufacturing Records (eBMR) are designed to simplify and automate batch record management in manufacturing environments. eBMR captures all batch-related data at every manufacturing process step, reducing errors and ensuring compliance.
Enhanced Batch Traceability: With eBMR, manufacturers can track every batch’s production history, ensuring that all steps meet regulatory requirements.
Real-Time Data Capture: eBMR automatically captures data during manufacturing, reducing the risk of human error.
Audit-Ready Documentation: eBMR ensures that batch records are always complete, accurate, and ready for audit inspection—whenever needed.
The everyday challenges of manual batch record management—such as data inconsistencies, audit delays, and incomplete records—are eradicated with eBMR. Its automated system ensures that each batch is perfectly documented, compliant, and ready for inspection.
How RIMS and eBMR Work Together to Create a Holistic Compliance Solution
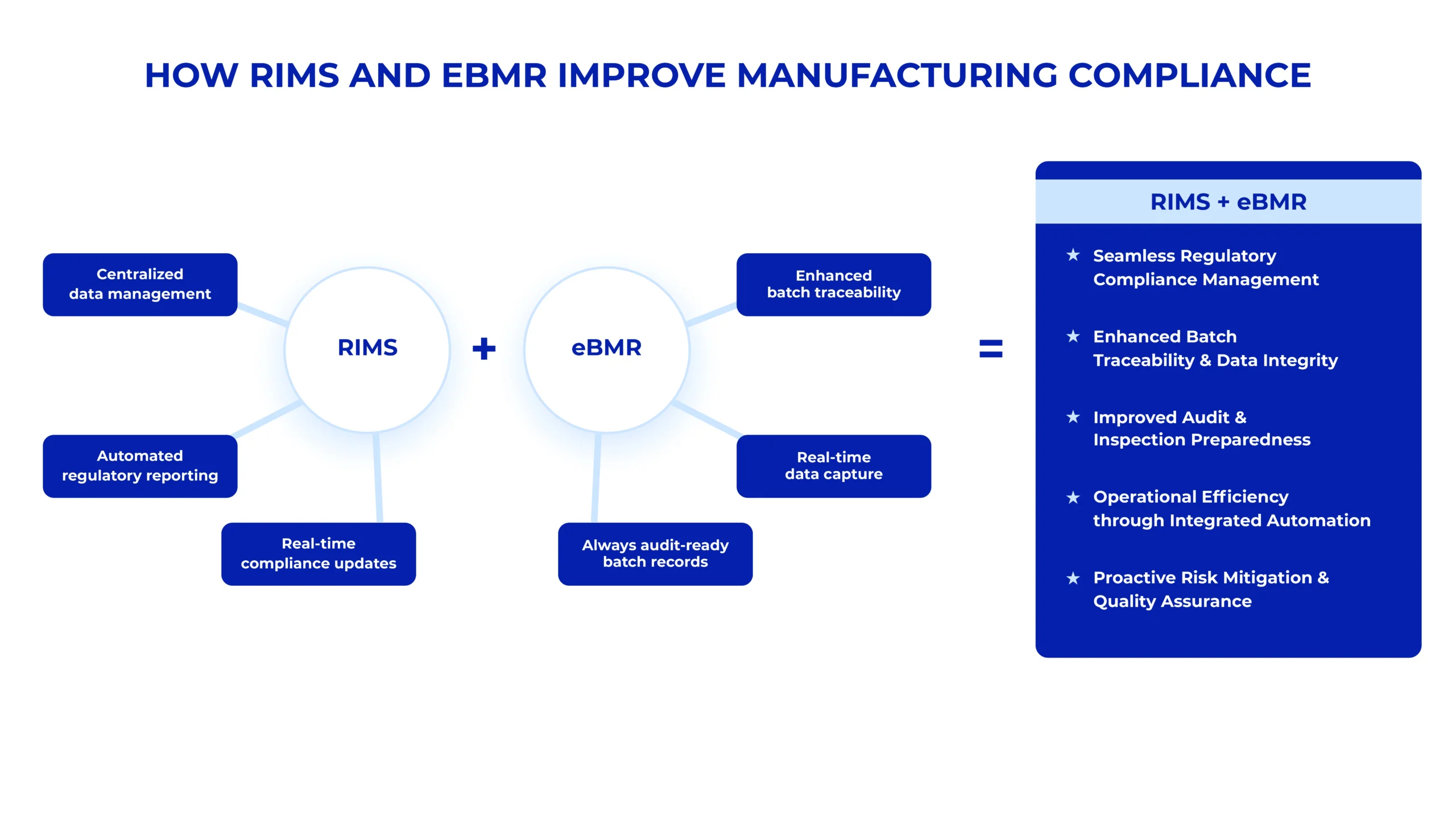
While both RIMS and eBMR are robust solutions, they offer a holistic, integrated approach to compliance management. Here’s how:
Data Synchronization: By seamlessly integrating RIMS and eBMR, AmpleLogic ensures data flows smoothly between systems. This eliminates information silos and ensures that every step of the manufacturing process—from initial production to final product release—is documented and compliant.
Seamless Compliance Tracking: With RIMS and eBMR in place, manufacturers can manage compliance from start to finish, tracking every aspect of the manufacturing process.
Enhanced Operational Efficiency: Integrating RIMS and eBMR means minimizing manual data entry, system switchovers, and redundant work. This allows staff to focus on more strategic tasks, such as improving manufacturing quality or driving innovation.
Integrating RIMS and eBMR creates a proactive, cohesive compliance strategy that empowers manufacturers to navigate the complexities of today’s regulatory landscape seamlessly.
Key Compliance Automation Benefits in the Manufacturing Sector
Compliance automation offers a host of benefits for the manufacturing sector, and AmpleLogic’s RIMS and eBMR systems are at the forefront of this revolution:
Reduced Human Error: By automating data capture and reporting, RIMS, and eBMR minimize the risk of human error, which is often a significant source of compliance issues.
Cost Savings: Automating compliance tasks reduces labor costs associated with manual record-keeping and administrative work. Additionally, it mitigates the risk of costly compliance penalties.
Improved Audit Readiness: RIMS and eBMR are designed to ensure manufacturers are always audit-ready. With these tools in place, manufacturers can reduce the time spent preparing for audits by up to 50%, ensuring that records are complete, organized, and compliant.
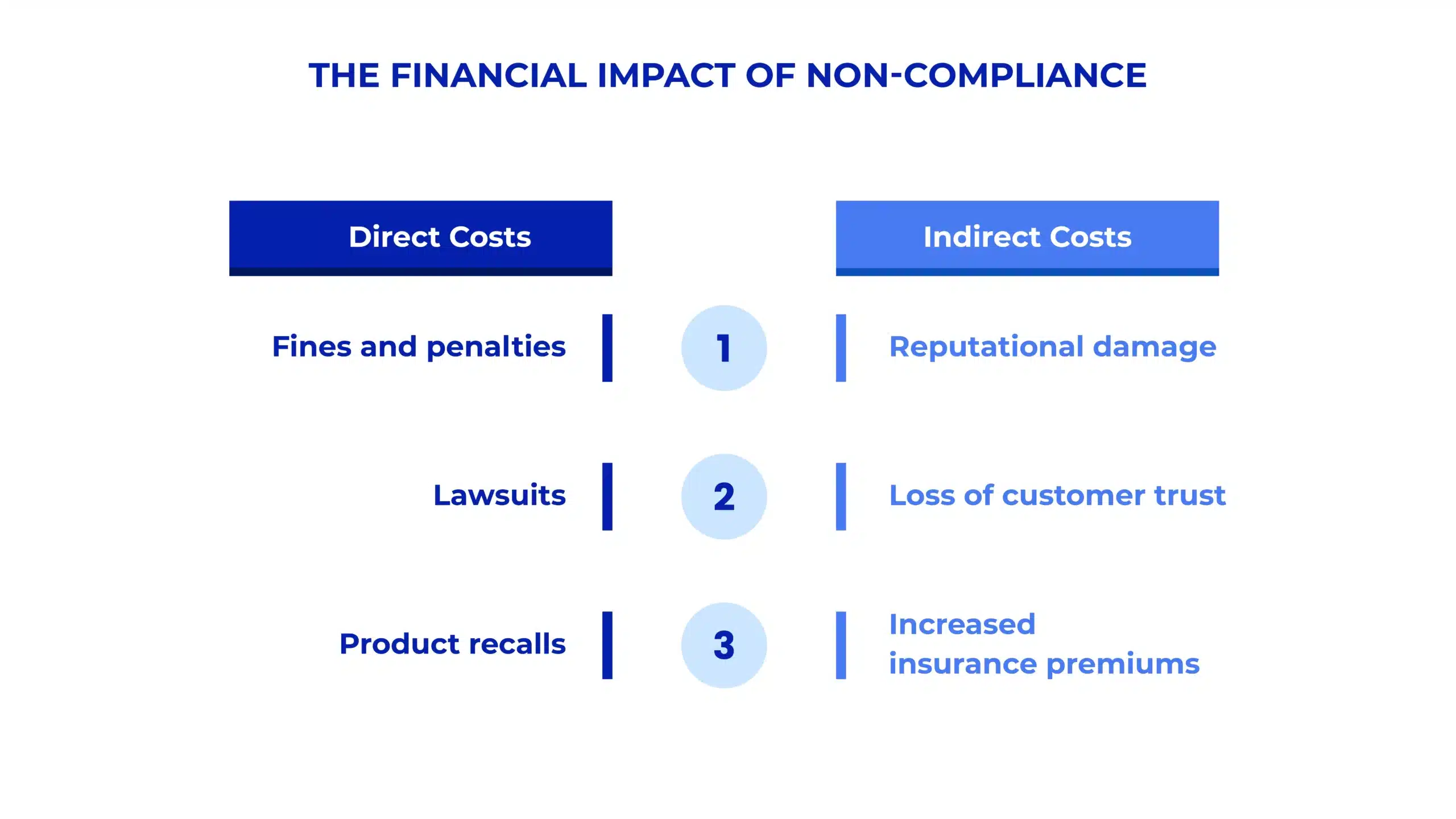
The Financial Impact of Non-Compliance in Manufacturing
Non-compliance in manufacturing can lead to significant financial consequences, ranging from fines and penalties to costly product recalls and lawsuits. The cost of non-compliance often goes beyond immediate financial penalties—it can also cause long-term damage to a company’s brand reputation and customer trust. Regulatory fines for missed deadlines, inaccurate record-keeping, and failure to meet industry standards can quickly cost millions of dollars, especially for global manufacturers.
Moreover, the risk of product recalls due to non-compliance with safety regulations or quality standards can lead to catastrophic losses. For instance, if a product fails to meet safety standards or regulatory approval, the recall process involves substantial logistics costs, reputational repair costs, and legal fees. Therefore, a proactive compliance strategy isn’t just about avoiding fines—it’s also about protecting the brand and securing a competitive advantage in the market.
Preparing for Audits and Inspections: Best Practices
Manufacturers must regularly prepare for audits and inspections to verify their compliance with industry regulations. Whether it’s an internal audit or an inspection by an external body, the process requires meticulous documentation and organization. An audit-ready manufacturer is likelier to pass inspections smoothly, avoid penalties, and demonstrate their commitment to regulatory standards.
Some best practices for audit preparation include:
- Regular internal audits to identify compliance gaps.
- Digitizing and centralizing records for easy access.
- Maintaining clear, accurate, and up-to-date records of every aspect of the manufacturing process.
- Training employees on how to respond to audits and ensuring they understand the importance of compliance.
- Working with compliance experts to anticipate potential issues and resolve them proactively.
An audit-ready organization is compliant and resilient to potential disruptions, safeguarding its operations and reputation.
The Impact of Global Regulations and Risk Mitigation Through Compliance Frameworks
As manufacturers expand their reach to international markets, they face the complex challenge of navigating various global regulations that vary by region. Compliance with international standards such as the European Union’s General Data Protection Regulation (GDPR) and the U.S. Food and Drug Administration’s (FDA) Good Manufacturing Practices (GMP) is critical, even for companies based outside these regions. These regulations often require manufacturers to adjust their processes, particularly in data management, product safety, and quality control.
Manufacturers must invest in specialized compliance solutions to successfully navigate this landscape and ensure their teams stay informed about evolving global regulations. This involves continuous education and an agile approach to adapting business practices to meet new rules.
A well-structured compliance framework helps manufacturers meet international regulatory demands and is an essential risk mitigation tool. Non-compliance can lead to significant financial penalties, product recalls, and damage to brand reputation. By implementing a robust compliance framework, manufacturers can proactively address these risks. This framework includes regularly auditing processes, maintaining accurate documentation, and staying updated on regulatory changes.
A compliance-first culture is central to minimizing risk. It encourages organizations to embed regulatory adherence in every aspect of their operations, from the factory floor to the boardroom. This proactive approach ensures that all employees, regardless of their role, understand the importance of compliance, reducing the chances of accidental violations and ensuring smooth business operations in global markets.
Why Manufacturers Trust AmpleLogic for Compliance Management
AmpleLogic has built a strong reputation as a trusted provider of compliance automation solutions, offering manufacturing companies a robust suite of tools to streamline and automate compliance management. However, what truly sets AmpleLogic apart is its commitment to customer success.
Many leading manufacturing companies have adopted RIMS and eBMR to transform their compliance processes. By using AmpleLogic’s solutions, they have improved operational efficiency and easily maintained regulatory compliance.
AmpleLogic’s comprehensive, expert-driven approach to compliance automation ensures that manufacturers can rely on their solutions for sustained compliance success, no matter how stringent the regulatory environment becomes.
Compliance remains a cornerstone of operational success as the manufacturing landscape evolves. With solutions like RIMS and eBMR, manufacturers can streamline their compliance management processes, reducing human error, improving efficiency, and ensuring regulatory adherence at every step. By embracing automation, manufacturers can stay ahead of the curve, ensuring their operations are always compliant and ready for the future.
As regulations continue to shift, these solutions are designed to be scalable and adaptable, ensuring manufacturers are equipped to navigate the complexities of future compliance challenges. Manufacturers looking to optimize their compliance processes and reduce operational costs should explore compliance automation solutions today—empowering their operations to meet the demands of tomorrow.






















