Managing Out of Specification (OOS) results in medical device manufacturing is very important for quality, regulatory compliance, and patient safety. The FDA and MHRA stress a scientific approach to OOS investigations, that is, that both a hypothesis-driven investigation and a thorough basis of documentation are combined in OOS investigations. Implementing a formal out of specification (OOS) procedure minimises compliance risks, avoids product recalls, and ensures consistency and reliability while manufacturing.
This article explores OOS definitions, regulatory requirements, investigation phases, and best practices for quality assurance. It also provides insights into how AmpleLogic, a leader in GAMP-compliant software solutions, enhances OOS management through advanced technology.
Out of Specification (OOS): Meaning and Implications
OOS stands for Out of Specification (for example, your test results don’t meet specifications for raw materials, in-process components, or finished medical devices). These variations are signals of potential quality issues that need to be further explored.
In microbiology, OOS (Out of Specification) results can have serious implications, such as product recalls, regulatory non-compliance, and patient safety risks. Alertness to OOS results also protects the integrity of the product (as well as compliance with industry standards) and prevents defective products from reaching the marketplace.
Must Read: Quality Management Systems (QMS) for Medical Devices
Common Causes of OOS in Medical Device Manufacturing
The common reasons for the occurrence of OOS during medical device manufacturing can be:
- Manufacturing Process Variability: Inconsistencies in raw materials or process parameters.
- Testing Errors: Analytical instrument malfunctions, human errors, or incorrect methodologies.
- Environmental Factors: Contamination, temperature, or humidity fluctuations affecting test outcomes.
- Deviations in Raw Materials: Poor quality or incorrect composition of components.
- Packaging and Labeling Issues: Mismatches in labeling or faulty packaging materials.
What is an Out of Specification (OOS) Result?
An OOS result occurs when a product’s test outcome (e.g., physical, chemical, or biological attribute) fails to meet predefined acceptance criteria. Examples include:
- A pacemaker battery exceeding voltage limits.
- Sterilisation cycles not achieving the required microbial reduction.
Must Read – QMS in Pharma: How AI is Transforming Quality Management
Regulatory Framework Governing OOS Procedures
The essential regulatory compliances for out of specification procedures are:
- FDA 21 CFR 820: Mandates documented procedures for nonconforming product investigations.
- MHRA Orange Guide: Requires root cause analysis (RCA) and corrective actions.
- ISO 13485:2016: Emphasizes risk-based decision-making during OOS resolution.
FDA and MHRA Guidelines
The FDA’s Guidance for Industry: Investigating Out of Specification Test Results for Pharmaceutical Production (updated in 2022) and the MHRA’s 2013 guidance outline structured approaches for OOS investigations. These frameworks mandate:
- Immediate Isolation: Quarantining affected batches to prevent further processing.
- Hypothesis-Driven Testing: Moving beyond repetitive retesting to identify root causes through scientific analysis.
- Documentation: Recording all investigative steps, results, and corrective actions to ensure traceability.
The regulatory requirements for OOS management under FDA 21 CFR 820, MHRA Orange Guide, and ISO 13485:2016 are complementary but distinct. FDA emphasises procedural rigor, MHRA prioritises RCA depth, and ISO 13485 integrates risk-based agility. Manufacturers aligning with these frameworks—supported by tools like eQMS and risk analytics—can mitigate compliance risks while enhancing operational efficiency.
AmpleLogic’s eQMS Software aligns with these requirements, offering automated compliance tracking and audit-ready documentation to streamline adherence.
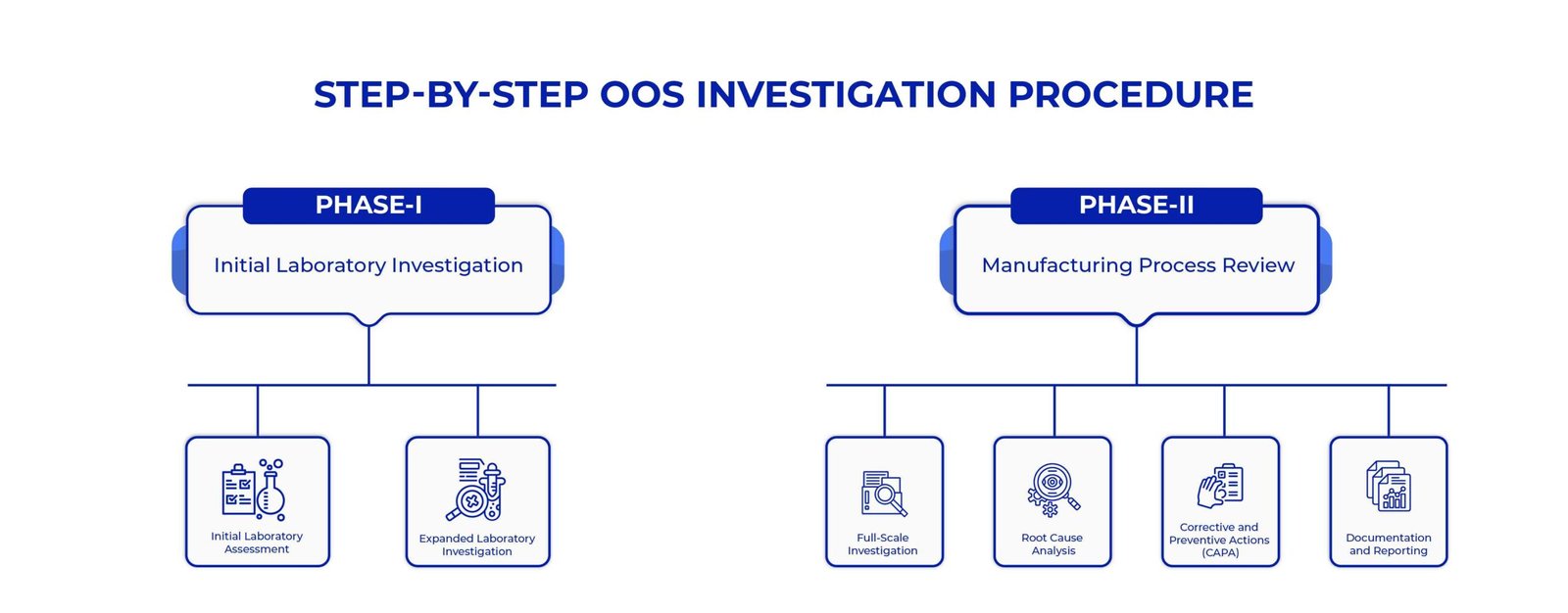
Step-by-Step OOS Investigation Procedure
A systematic and disciplined methodology for OOS investigations is crucial to discovering root causes and developing solutions. Here is a step-by-step investigation procedure for a solid OOS investigation framework:
Phase 1: Initial Laboratory Investigation
- Initial Laboratory Assessment
- Data Verification: Confirm the accuracy of the test data, ensuring proper instrument calibration and adherence to analytical methods.
- Analyst Review: Evaluate analyst performance to rule out human error, including potential sample mishandling or miscalculations.
- Expanded Laboratory Investigation
- Re-testing Protocol: Conduct re-testing to determine if the OOS result persists, ensuring that re-testing is justified and scientifically valid.
- Methodology Review: Assess the suitability and robustness of the analytical methods employed, making adjustments as necessary.
Phase 2: Manufacturing Process Review
- Full-Scale Investigation
- Production Process Examination: Investigate manufacturing processes for deviations or anomalies that could contribute to OOS results.
- Material Quality Assessment: Evaluate raw materials and components for conformity to quality standards, identifying potential sources of variation.
- Root Cause Analysis
- Analytical Tools Application: Utilize tools such as Fishbone Diagrams, 5-Whys Analysis, or Failure Mode and Effects Analysis (FMEA) to pinpoint the underlying cause of the OOS result.
- Corrective and Preventive Actions (CAPA)
- Immediate Corrections: Address the identified root cause promptly to prevent recurrence.
- Preventive Strategies: Implement long-term measures, such as process optimisation or enhanced training programs, to mitigate future risks.
- Documentation and Reporting
- Comprehensive Record-Keeping: Document all aspects of the investigation, including findings, actions taken, and rationale for decisions, ensuring transparency and traceability.
- Regulatory Communication: Report significant findings to regulatory authorities as required, maintaining compliance with current guidelines.
AmpleLogic integrates with CAPA (Corrective and Preventive Action) and LIMS (Laboratory Information Management Systems) to streamline root cause analysis and ensure seamless cross-departmental collaboration during Phase II.
OOS vs. Deviations: Key Differences
While out of specification procedure results focus on analytical failures, deviations refer to unplanned departures from approved manufacturing protocols.
For example, an OOS event could involve a sterilised device exceeding microbial limits, whereas a deviation might occur if an autoclave cycle is executed at incorrect temperatures. Both scenarios require investigations but differ in scope: OOS addresses product quality, while deviations address process integrity.
Below is a table showcasing the difference between OOS and Deviation:
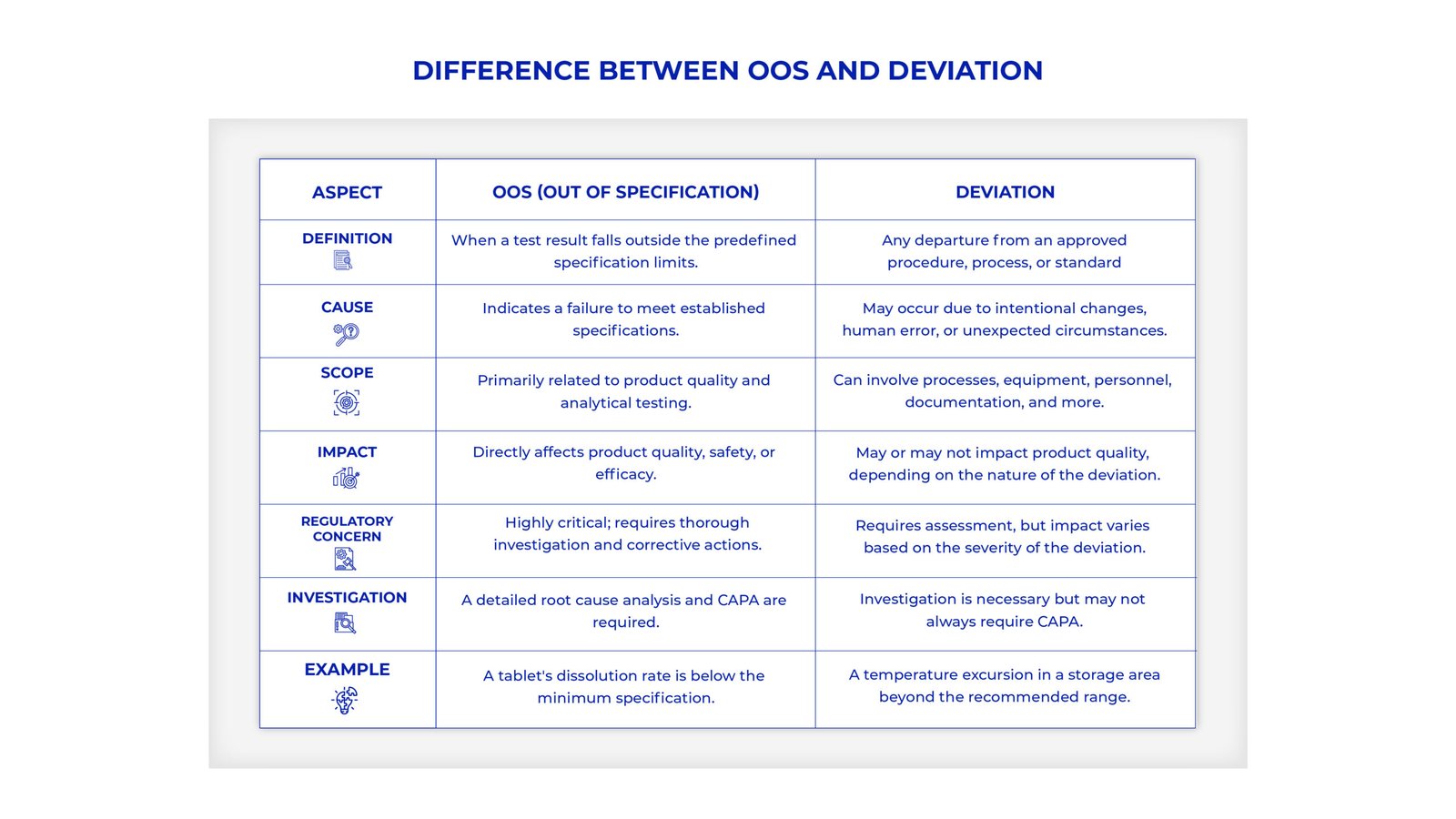
| Criteria | OOS | Deviation |
|---|---|---|
| Focus | Product quality failure | Process nonconformance |
| Example | Failed tensile strength test | Skipped cleaning validation step |
| Resolution | Retesting + RCA | Process correction + Documentation |
7 Best Practices for Effective OOS Resolution
Here are some best practices that can be performed for an effective OOS resolution –
- Root Cause Analysis (RCA): Deploy tools like Fishbone diagrams or 5 Whys to systematically identify failure sources.
- Corrective and Preventive Actions (CAPA): Address both immediate causes (e.g., recalibrating equipment) and systemic gaps (e.g., updating training programs).
- Data Integrity: Ensure electronic records comply with ALCOA+ principles (Attributable, Legible, Contemporaneous, Original, Accurate).
- Standardised Testing Procedures: Follow validated analytical methods to minimise testing errors.
- Regular Equipment Calibration: Ensure testing instruments are regularly calibrated and maintained.
- Employee Training Programs: Conduct periodic training for personnel on quality control protocols.
- Robust Supplier Quality Management: Evaluate and monitor suppliers to ensure material consistency.
With AmpleLogic’s AI eQMS, organisations can proactively prevent OOS deviations by digitising workflows and enforcing best practices across all manufacturing operations.
Conclusion
Out of Specification procedures are indispensable in medical device manufacturing, ensuring products meet safety and efficacy standards. Manufacturers can mitigate risks, enhance compliance, and uphold patient trust by adhering to regulatory guidelines, employing evidence-based investigations, and leveraging AmpleLogic’s advanced OOS Software. Integrating scalable digital solutions remains critical for operational excellence as regulatory landscapes evolve.
AmpleLogic’s blend of innovation, compliance expertise, and customer-centric partnerships positions it as a strategic ally for medical device manufacturers navigating the complexities of OOS management. Explore AmpleLogic today to see how it can transform your software experience. Also, you can visit our page for other informative articles, such as AmpleLogic Resources!