
A growing list of data integrity warnings issued to pharmaceutical companies has prompted an urgent focus on strengthening tracking mechanisms to prevent process failures. One key solution is digitization, creating a centralized data repository to make information easily accessible for analyzing issues and predicting challenges.
However, digital transformation across pharma is progressing more slowly than expected, leading to extended involvement of quality teams. Implementing pre-validated software such as LIMS, DMS, QMS, and LMS often exceeds 12 months, while electronic Batch Manufacturing Records (eBMR) can take more than three years. These timelines frequently overshoot due to rework and mid-project requirement changes.
This domino effect disrupts documentation efforts, requiring frequent updates and revision cycles. To reduce these complications, flexible and agile implementation methods are critical. Notably, Operational Qualification (OQ) alone consumes nearly 60% of the total project effort. This raises a crucial question: How effective is this for configurable software (GAMP Category 4 & 5)? Despite large investments, expected outcomes often fall short.
Key Challenges
From the Pharma Industry Side:
- Poor documentation of business user requirements
- Frequent turnover in senior management
- Lack of detailed functional requirements with traceability to user needs
- High costs tied to change requests
- Absence of an up-to-date traceability matrix
From the Supplier Side:
- Rigid systems requiring code for every major change
- Limited domain knowledge and under-skilled programmers
- Reliance on outdated software implementation methodologies
V Model: Verification and Validation Model
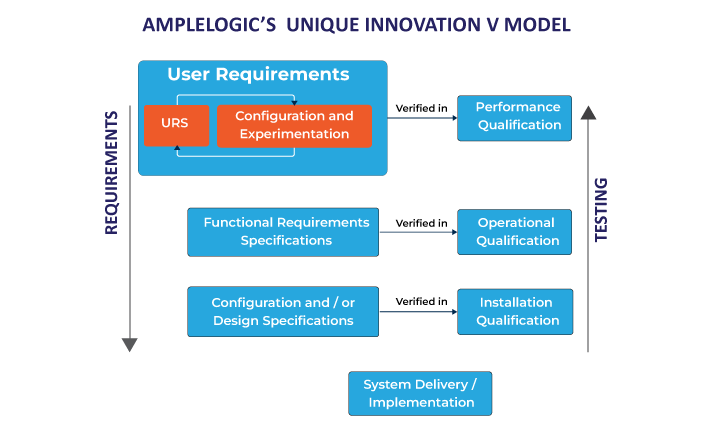
The V-Model remains a common framework for software implementation in pharma, relying on clearly defined requirements and acceptance criteria. However, the time gap between User Requirement Specification (URS) and User Acceptance Testing (UAT) is long, and during that time, requirements often evolve.
Because users don’t see a working system until late in the process, many change requests only surface at the end. Updating test plans and requirement documents to reflect these mid-cycle changes adds cost and delay. Major gaps found during OQ can be prohibitively expensive and derail the validation timeline.
Over the past decade, regulatory inspections have increasingly scrutinized software validation across all stages from specification writing and risk assessment to IQ/OQ/PQ, revalidation, reporting, and change control.
The Traditional V-Model Must Be Revisited
Configuration and Experimentation Phase
A key improvement is the inclusion of a Configuration and Experimentation phase between the URS and Functional Requirement Specification (FRS) stages. This enables business users to interact with pre-validated software and align it with their actual use cases early in the project.
This hands-on phase helps refine requirements before FRS finalization, ensuring clarity on software outcomes. It also addresses common regulatory audit findings related to revalidation, deviation management, and release versioning.
However, this iterative approach cannot be supported by traditional configurable software, it requires platforms with visual modeling capabilities, such as No Code/Low Code Development Platforms. These allow users to visualize software creation as it happens.
In 2025, regulatory bodies like the FDA and EMA have intensified their scrutiny of data integrity, demanding robust audit trails, strict access controls, and validated data processes. Companies must now implement Continuous Process Verification (CPV) to monitor and control manufacturing in real time across the product lifecycle, ensuring consistent quality and compliance.
No Code/Low Code Platforms: The Agile Alternative
No-code/low-code platforms allow business and IT teams to collaborate through visual modeling. Requirements are captured clearly, and applications can be built, tested, and iterated in days and not months.
These platforms provide:
- Visual modeling of logic and workflows (with custom code extensibility)
- Visual data modeling
- Drag-and-drop interfaces for multi-device UI development
- Application change management and lifecycle support
They empower both developers and non-developers to quickly build customized workflows and applications, boosting productivity and minimizing errors.
European Health Data Space (EHDS) Regulation
The EU’s EHDS regulation, rolled out in March 2025, mandates standardized electronic health records and cross-border interoperability. This underscores the need for flexible digital platforms like No Code/Low Code to meet evolving data integrity and compliance expectations.
The Way Forward for Pharma Organizations & IT Organizations
Pharmaceutical companies should now consider pre-validated software built on visual modeling capabilities (Low Code/No Code platforms). These platforms enable intuitive, human-readable application development while keeping costs consistent.
Moreover, ongoing staff training on data integrity, as reinforced by recent FDA and EMA guidelines, will be crucial for sustained compliance and operational excellence.
Software vendors and pharmaceutical IT teams must shift focus from documentation-heavy processes to visual modeling and rapid prototyping. By investing in No-Code/Low-Code platforms, IT teams can deliver scalable, complex applications faster with fewer resources.
Schedule a free demo now to see how our low-code platforms can simplify your compliance process. Interested in additional information? Check out our resources page for detailed articles and insights.






















