
Pharmaceutical companies and R&D labs today find themselves navigating a complex landscape, caught between the need for relentless innovation, improved operational efficiency, and staying ahead of market trends, all while ensuring strict adherence to regulatory frameworks. R&D labs, in particular, face the added challenge of managing compliance and maintaining data integrity, all while rapidly shifting focus between projects or scaling operations based on evolving research outcomes.
To tackle these multifaceted challenges, many pharmaceutical organizations are embracing Digital Transformation, leveraging advanced technologies to automate, scale, and optimize their processes. As cloud computing continues to reshape the industry, SaaS (Software as a Service) and Low-code aPaaS (Application Platform as a Service) have emerged as the leading solutions. In this article we discuss the key technological, regulatory, and integration requirements of the pharmaceutical sector, examining which solution SaaS or Low-code aPaaS (with pre-built applications) has the potential to create the greatest impact in addressing these critical industry needs.
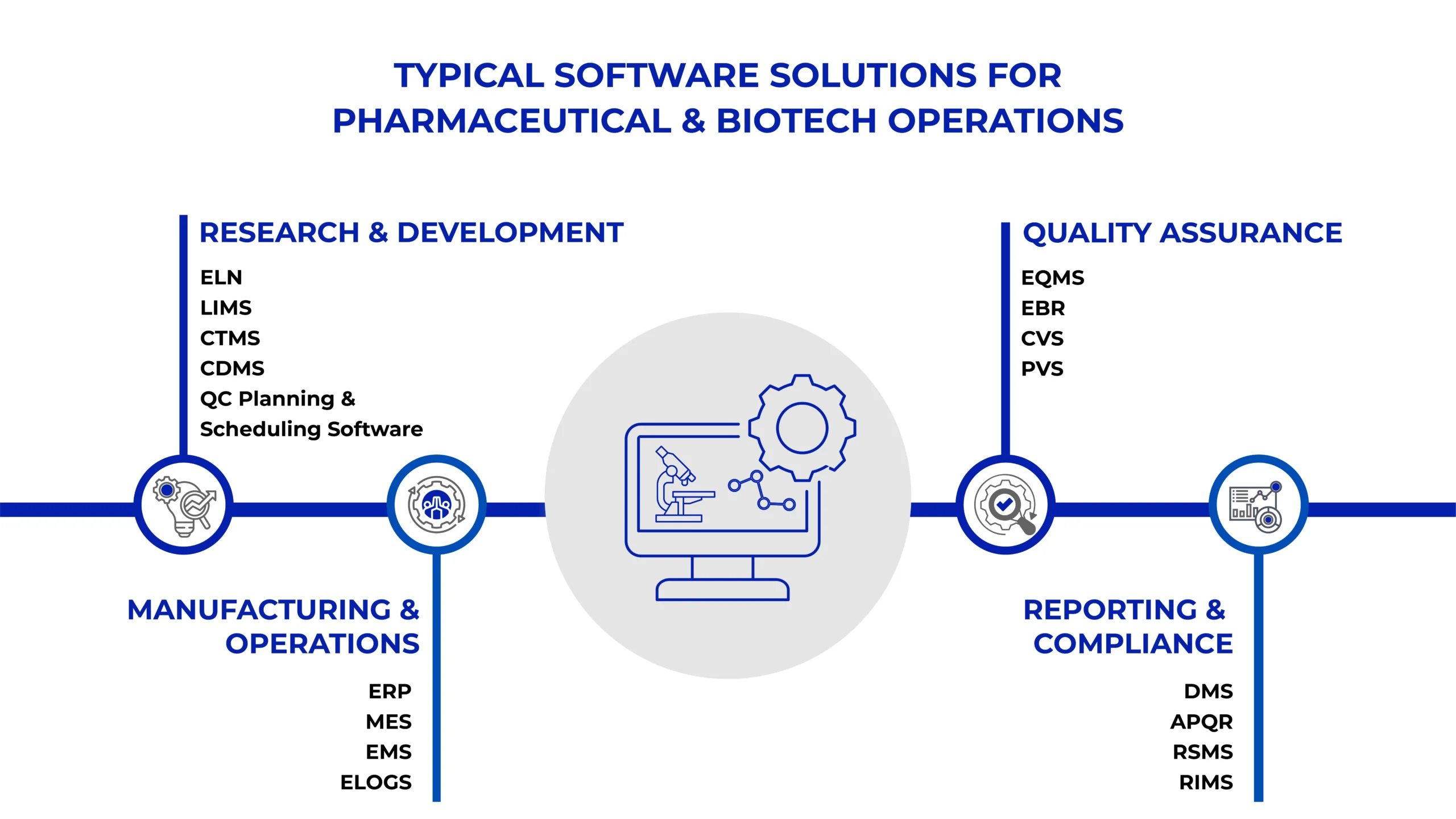
Typical Software Solutions for Pharmaceutical & Biotech Operations
Pharmaceutical companies require a broad suite of software applications to manage operations, ensure compliance, and drive efficiency. These applications typically fall into the following categories:
Manufacturing and Operations: ERP systems manage non-GMP operations, MES enables real-time production monitoring, and Equipment Management Systems handle calibration and maintenance. Cleaning Validation minimizes cross-contamination risks during manufacturing, while eLogbook and Electronic Lab Notebooks manage R&D lab data and experiments, ensuring data integrity
Compliance and Quality Management: EQMS manages SOPs, change control, deviations, CAPA, and training, ensuring adherence to regulatory standards such as GxP and FDA 21 CFR Part 11
Research and Development: Electronic Lab Notebooks (ELNs) and LIMS captures R&D data, ensuring data integrity and maintaining compliance with industry regulations
Clinical Trials: Clinical Trial Management Systems plan, track, and manage trial data, ensuring robust documentation for regulatory bodies
Business Process Excellence: APQR automates data collection from ERP, MES, QMS, and LIMS for trend analysis, statistical analysis and report generation to maintain high product quality and regulatory compliance

Essential GxP and Data Integrity Standards
Software used in regulated sectors such as lifesciences, pharmaceutical, biotech, food & beverages, cosmetics and other industries need to be compliant with GxP and data integrity standards. Some of these requirements are mentioned below.
Regulatory Compliance: The software should help organizations comply with global regulations such as 21 CFR Part 11, GxP, etc put forward by US FDA, EU EMA, and other regulatory bodies
Audit Trails and Traceability: Such software must maintain secure records of all activities with timestamps, user details, and the ability to retrieve historical data for audits
Data Integrity: The software should provide accurate and complete data that is protected against tampering
Growing Need for Seamless Integration
For meeting compliance, promoting innovation, and streamling operations, integration across various applications is essential in the pharmaceutical sector. Integration plays an important role while dealing with the overall operations in pharma. It influences business goals, technological stack and the legal requirements. In addition to integration between applications, there is a need to integrate AI decision-making features in such software.
For instance, the eLogbook cleaning module and LIMS sample analysis can be used to track cleaning validation failure, which immediately initiates the cleaning operator’s training in the LMS.
Key integrations that are frequently required in pharmaceuticals are listed below:
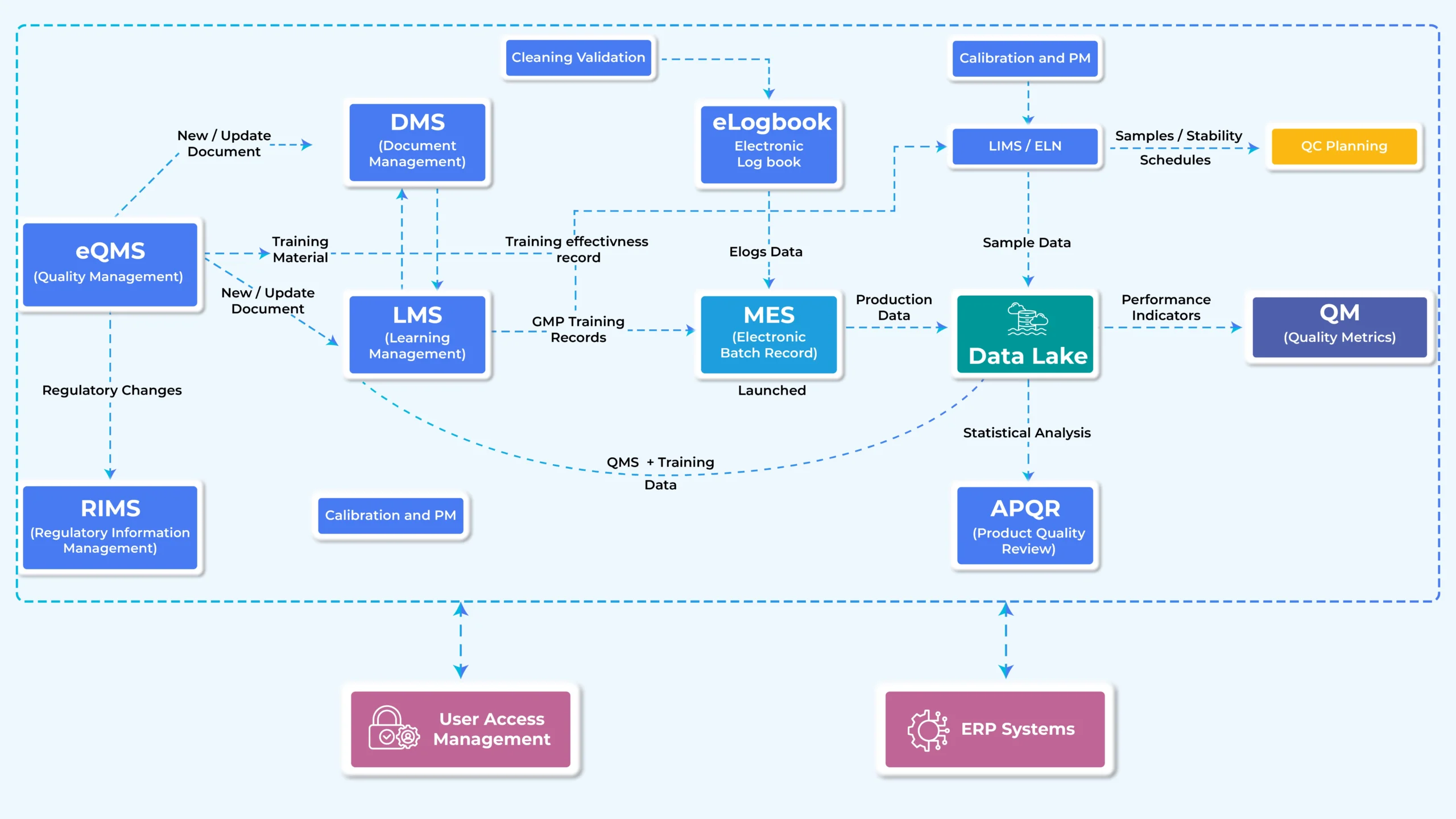
A critical first step in increasing operational effectiveness, boosting regulatory compliance, and stimulating innovation in the pharmaceutical sector is selecting the appropriate software for digital transformation.
Considerations for Choosing the Right Software
- Domain-specific solution that complies with regulatory standards like FDA 21 CFR Part 11, HIPAA, and GDPR
- Integration with current systems and standard integration among pre-built applications
- Easy migration from legacy systems
- Customization benefits
- Support to data lakes
- Future readiness for Personalized Medicine
- Able to support On-Premises, Private Cloud and Hybrid Deployments to provide greater control over data and security
- Able to automate certain tasks in application using AI
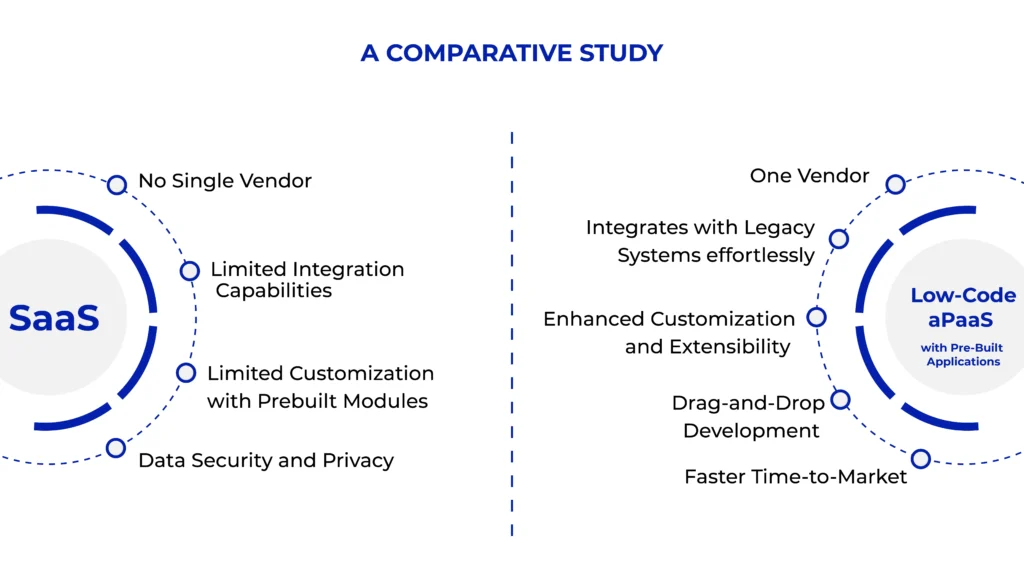
Is SaaS a correct choice?
- No Single Vendor: Single SaaS vendor for multiple applications is often rare
- Integration with Legacy Systems: Many lifesciences companies operate with legacy on-premise systems that may not seamlessly integrate with modern SaaS solutions
- Prebuilt modules: SaaS platforms often provide standardized solutions, which may not fully meet the specific needs of lifesciences companies
- Vendor Lock-In: Switching SaaS providers can be difficult due to proprietary systems, data migration challenges, and high costs
- Data Security and Privacy: Lifesciences companies handle sensitive data, such as patient information and proprietary research data, which must be safeguarded against breaches
- Customization Limitations: SaaS platforms often provide standardized solutions, which may not fully meet the specific needs of lifesciences companies
Is Low-Code aPaaS with Pre-Built Applications the right choice?
Businesses, especially those in the pharmaceutical sector, seeking to quickly develop, deploy, and customize applications with less coding can consider a Low-Code aPaaS (Application Platform as a Service) with pre-built applications. These platforms speed up development time and cut costs by giving non-technical users the ability to create or improve programs using visual development tools, reusable components, and templates. Low-Code aPaaS makes it easier to develop applications for clinical trials, manufacturing, supply chain management, quality and regulatory compliance, and other areas in the pharmaceutical sector.
Drag-and-Drop Development: Instead of writing a lot of code, users may develop and launch programs by only dragging and dropping components
Integration Capabilities: Comes with built-in connectors for common systems used in pharma and for easy integration
Customization and Extensibility: Allows a great deal of customisation although being low-code When necessary, developers can expand the platform’s functionality by adding custom code (such as intricate computations or connections with specialist pharmaceutical systems).
Faster Time-to-Market: Pharmaceutical firms can deploy applications more rapidly by using pre-built apps and reusable components, which shortens the time it takes to move from concept to execution. Reduces validation timelines.
Pharmaceutical companies must balance innovation with regulatory compliance. While SaaS offers standardized solutions, it presents challenges like limited customization, vendor lock-in, and integration issues. Low-Code aPaaS platform with pre-built application provide flexibility, faster development, and seamless integration, making them ideal for regulated industries. With embedded compliance features like FDA 21 CFR Part 11 and GDPR, the platform reduces regulatory burdens and accelerates time-to-market. Adopting Low-Code aPaaS with pre-built applications ensures cost-effective scalability and future readiness, enabling pharmaceutical companies to achieve operational excellence in a dynamic, highly regulated environment.





















