Maintaining integrity in batch records is crucial in pharmaceutical manufacturing for compliance and quality assurance. Traditional paper-based methods often pose challenges in terms of accuracy, efficiency, and regulatory adherence. The advent of Manufacturing Execution Systems (MES) in pharma industry has revolutionized the way companies manage batch records, offering advanced solutions to streamline processes and ensure compliance with industry standards.
The Challenge of Manual Batch Record Management in Pharmaceutical Manufacturing:
Manual Processes: Traditional paper-based batch record systems are prone to human errors, transcription mistakes, and inefficiencies. The manual entry of data increases the risk of errors and inconsistencies, leading to potential quality issues and compliance violations.
Compliance Challenges: Meeting regulatory requirements, including FDA regulations such as 21 CFR Part 11, can be daunting with manual systems. Paper-based processes make it difficult to maintain data integrity, track changes, and provide the necessary documentation to regulatory authorities.
Limited Visibility: Lack of real-time monitoring and traceability hinders the ability to identify and address discrepancies promptly. Without visibility into production processes, manufacturers may struggle to detect deviations from standard procedures, jeopardizing product quality and regulatory compliance.
Inefficient Production Planning: Manual production planning and scheduling often lead to suboptimal resource utilization and prolonged cycle times. Without automated tools to optimize production schedules and allocate resources efficiently, pharmaceutical companies may face delays, excess inventory, and increased costs.
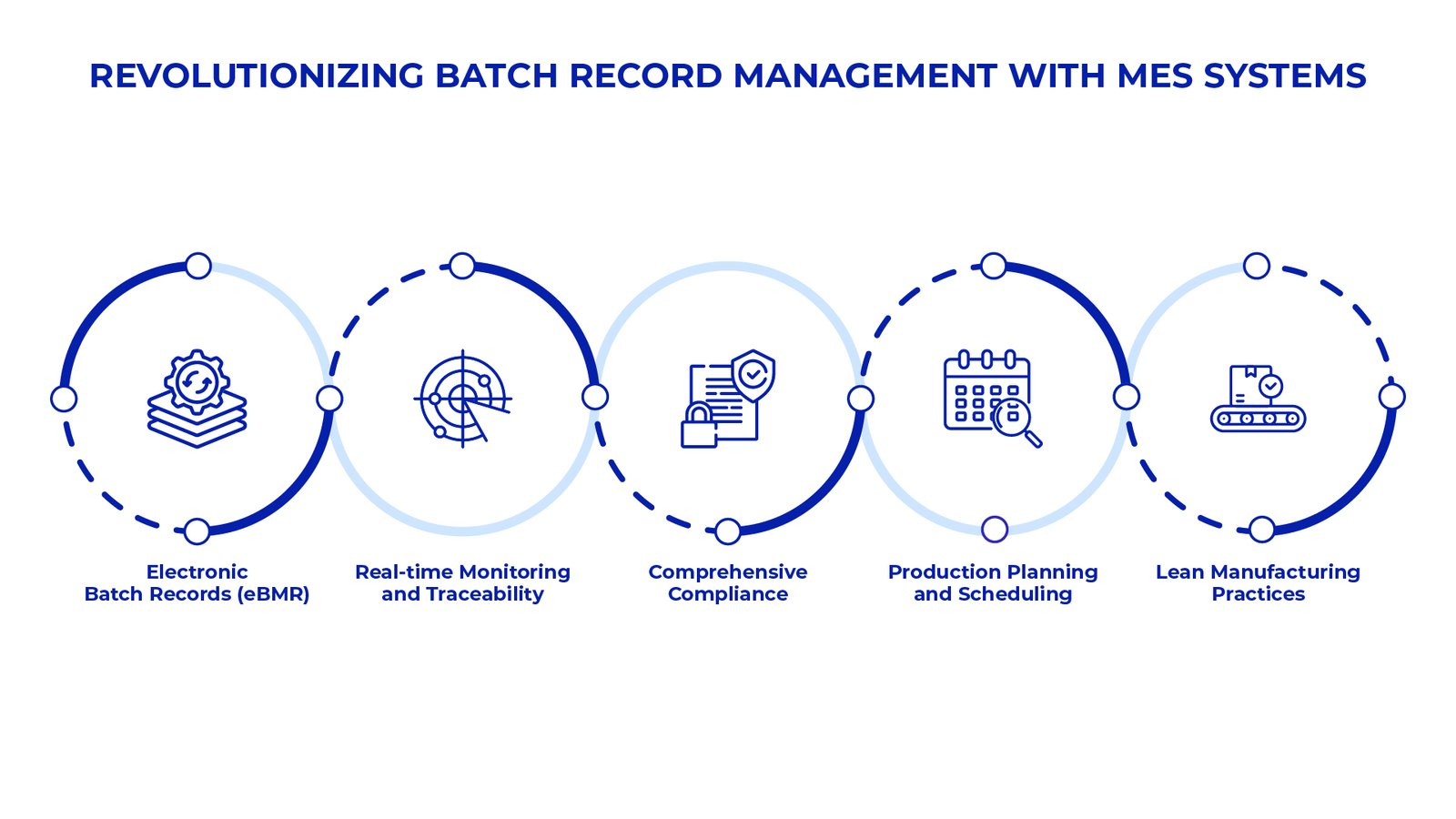
Revolutionizing Batch Record Management with MES Systems:
Electronic Batch Records (eBMR): Implementing an eBMR system digitizes the entire batch record process, ensuring accuracy and consistency in data capture and management. Electronic records eliminate the need for manual data entry, reducing the risk of errors and facilitating real-time access to critical production information.
Real-time Monitoring and Traceability: MES for pharma manufacturing offers real-time visibility into production processes, enabling timely identification and resolution of discrepancies. Advanced traceability features track the entire manufacturing history of each batch, ensuring transparency and compliance with regulatory requirements.
Comprehensive Compliance: MES for pharmaceutical manufacturing is designed to meet regulatory requirements, incorporating electronic signatures, audit trails, and data integrity measures to ensure compliance with industry standards. Automated validation processes streamline compliance efforts, reducing the burden on manufacturing teams and minimizing the risk of non-compliance.
Production Planning and Scheduling: Automated production planning and scheduling algorithms optimize resource utilization, minimize downtime, and ensure on-time delivery of products. By analyzing production data in real-time, MES systems in pharma enable proactive decision-making, improving efficiency and reducing lead times.
Lean Manufacturing Practices: MES systems in pharma support lean manufacturing principles by eliminating waste, reducing cycle times, and improving overall efficiency through automation and streamlined workflows. By standardizing processes and reducing variability, pharmaceutical companies can achieve higher levels of productivity and quality while minimizing costs.
In conclusion, adopting MES systems like AmpleLogic MES is essential for pharmaceutical companies looking to address batch record discrepancies, ensure accuracy, and maintain compliance with regulatory standards. By leveraging advanced technology and automation, these systems empower manufacturers to streamline operations, enhance productivity, and uphold the highest standards of quality and safety. With a focus on innovation and customer satisfaction, AmpleLogic remains at the forefront by providing robust MES solutions for pharmaceutical manufacturing.