Embrace AmpleLogic's GAMP Solutions for Compliance Success
At AmpleLogic, we believe in making quality a seamless part of your everyday operations. With just one click, embark on a journey where quality becomes a habit, not just an act. Explore our diverse range of 12 applications, meticulously designed to ensure compliance with the rigorous standards set forth by regulatory bodies such as the USFDA and MHRA.
In the ever-evolving landscape of regulatory compliance, industries such as pharmaceuticals, biotechnology, and healthcare face significant challenges in maintaining adherence to stringent guidelines. The Good Automated Manufacturing Practice (GAMP) framework has emerged as a beacon of assurance, providing structured guidance for the validation of automated systems. AmpleLogic, a leading provider of innovative software solutions, has embarked on a mission to revolutionize compliance with its cutting-edge GAMP Solutions.
Revolutionizing Compliance with GAMP Solutions by AmpleLogic
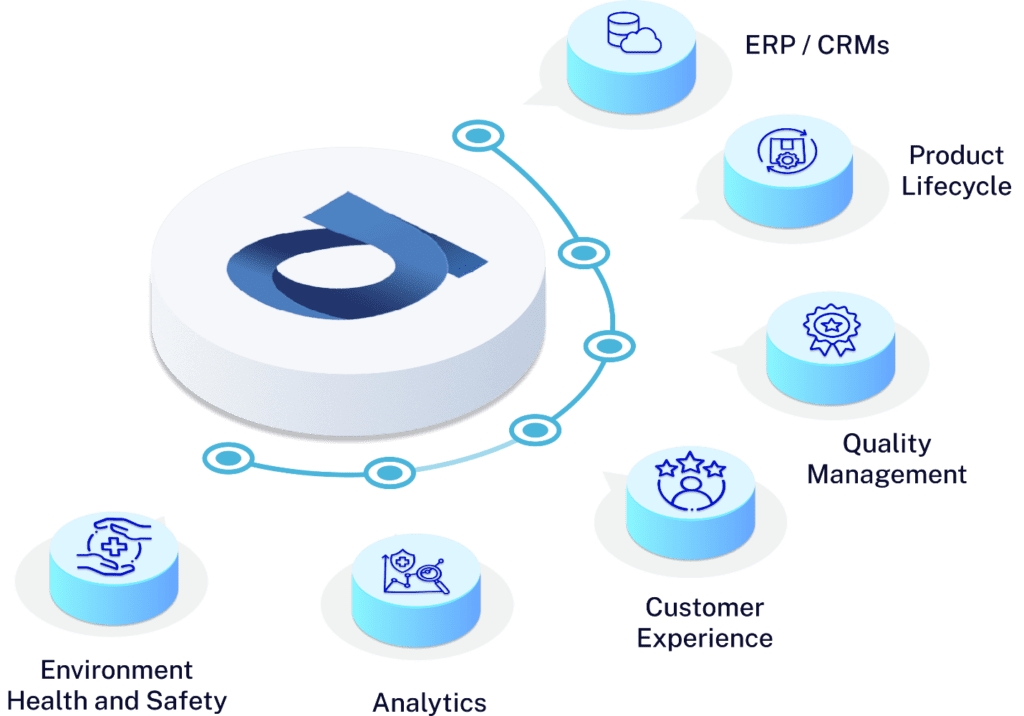
Seamless Integration with World's Finest Technologies
Benefits of AmpleLogic's GAMP Solutions

Enhanced Compliance
Achieve and maintain compliance with confidence, supported by robust validation methodologies and best practices.

Enhanced Compliance
Achieve and maintain compliance with confidence, supported by robust validation methodologies and best practices.
Operational Efficiency
Drive efficiency gains across validation processes, optimizing resource utilization and accelerating time-to-market.

Operational Efficiency
Drive efficiency gains across validation processes, optimizing resource utilization and accelerating time-to-market.

Risk Mitigation
Minimize compliance risks through systematic documentation management, real-time monitoring, and proactive risk assessment.

Risk Mitigation
Minimize compliance risks through systematic documentation management, real-time monitoring, and proactive risk assessment.
Cost Savings
Reduce validation costs and overheads associated with manual processes, while maximizing ROI through streamlined operations.

Cost Savings
Reduce validation costs and overheads associated with manual processes, while maximizing ROI through streamlined operations.
Competitive Advantage
Gain a competitive edge by leveraging innovative GAMP Solutions that foster agility, innovation, and regulatory excellence.

Competitive Advantage
Gain a competitive edge by leveraging innovative GAMP Solutions that foster agility, innovation, and regulatory excellence.
Hear From Our Customers
Contact us
Your Pharma Automation Starts Here
We’re here to address your inquiries and assist you in identifying the solutions that best align with your requirements. Here’s why choosing us is your strategic advantage:
Your benefits:
- Client-oriented
- Independent
- Competent
- Results-driven
- Problem-solving
- Transparent
What happens next?
1
Schedule a call at your convenience
2
Discovery and consultation session
3
Get your custom proposal


























