
In the pharmaceutical realm, upholding stringent standards of quality and regulatory compliance stands as a backbone in guaranteeing the safety and efficacy of medicinal offerings. Among the pivotal processes mandated by regulatory bodies like the FDA and EMA, the Annual Product Quality Review (APQR) emerges as a cornerstone. This rigorous evaluation delves deep into product quality data, aiming not just for consistency but also to pinpoint trends and catalyze continuous improvement initiatives. Within this narrative, we delve into the profound significance of APQR and illuminate how the AmpleLogic APQR software serves as a catalyst, empowering pharmaceutical enterprises to attain business process excellence and unwavering regulatory adherence.
Understanding Annual Product Quality Review
Annual Product Quality Review (APQR) is a systematic review of product quality data conducted annually by pharmaceutical manufacturers. Its primary objective is to evaluate the quality, safety, and efficacy of medicinal products throughout their lifecycle. Annual product quality review encompasses a wide range of data, including manufacturing processes, specifications, deviations, complaints, stability data, and analytical results. By analyzing this data, manufacturers can identify trends, deviations, and areas for improvement, ensuring compliance with regulatory standards and driving continuous improvement in product quality.
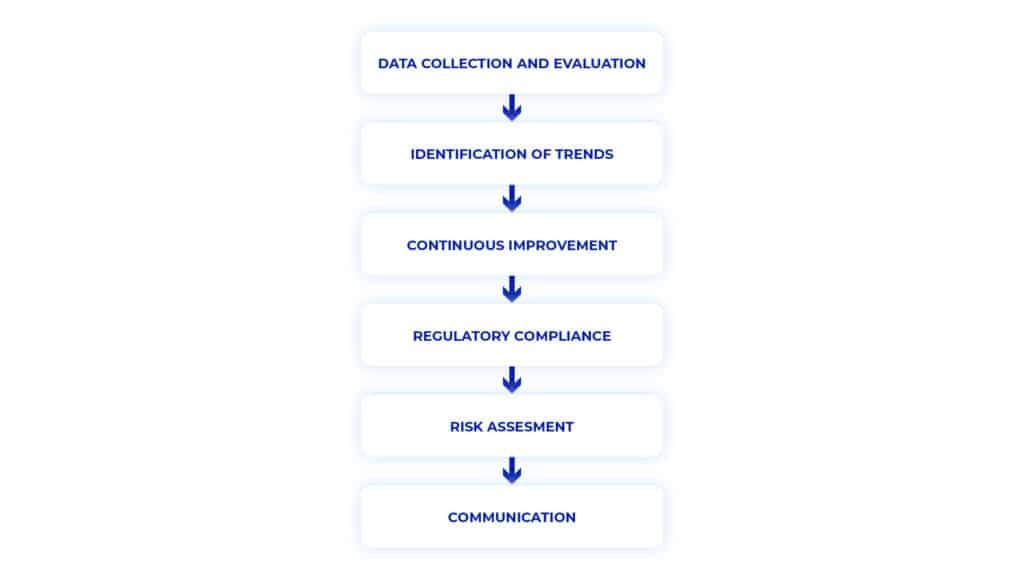
Importance of Annual Product Quality Review
APQR plays a crucial role in pharmaceutical manufacturing for several reasons:
Regulatory Compliance: Mandated by agencies like the FDA and EMA, showcases a company’s dedication to quality management and regulatory adherence.
Quality Assurance: Maintaining consistent product quality, safety, and efficacy. Identification of deviations enables proactive measures for product quality and patient well-being.
Continuous Improvement: Facilitates ongoing enhancement of manufacturing processes. Data analysis allows companies to optimize operations, improving efficiency and minimizing quality risks.
Risk Management: Allows systematic risk assessment, helping identify vulnerabilities and implement proactive measures to prevent quality issues and regulatory non-compliance.
Data-driven Decision Making: Involves comprehensive data analysis, empowering informed decisions on product quality, process optimization, and regulatory compliance.
Continuous Compliance Monitoring: Enables ongoing monitoring of product quality and regulatory compliance, reducing the risk of recalls or regulatory actions.
Quality System Evaluation: Assesses the effectiveness of the quality system, identifying strengths and weaknesses to implement necessary corrective actions.
Documentation and Traceability: Mandates documenting findings and actions, ensuring a comprehensive record for regulatory compliance, audits, and inspections.
Introducing AmpleLogic APQR Software
AmpleLogic Annual product quality review software is a comprehensive solution designed to streamline and automate the process. It offers a range of features and capabilities to help pharmaceutical companies achieve business process excellence and regulatory compliance:
Data Integration and Aggregation: Integrates diverse data sources like manufacturing systems and quality control labs in real-time, ensuring data integrity and streamlining processes.
Automated Reporting and Analysis: Automates report generation and provides advanced analytics to identify quality trends and root causes efficiently, enabling informed decision-making and continuous improvement.
Risk-Based Approach: Prioritizes resources based on areas of highest risk to product quality and patient safety, with tools for risk assessment and proactive risk mitigation.
Integration Capabilities: Breaks down data silos between manufacturing, quality control, and supply chain data, enhancing holistic insights and decision-making.
Cross-Functional Department Collaboration: Facilitates collaborative processes, allowing users to contribute, approve, and comment on reports seamlessly.
Continued Process Verification (CPV): Provides continuous monitoring of critical parameters throughout the manufacturing process, ensuring ongoing compliance and quality assurance.
Compliance against 3 and 6 Sigma: Aligns with statistical process control methodologies, ensuring precise and reliable product quality assessments.
Golden Batch Facilitation: Supports identifying and replicating optimal manufacturing conditions consistently for quality improvement.
Regulatory Compliance Management: Equipped with compliance features like e-signatures and audit trials as per regulatory requirements, ensuring seamless adherence to standards like 21 CFR Part 11 and EU Annex 11.

Case Studies and Success Stories
Bharat Serums and Vaccines has been a renowned name in the lifesciences industry. The company has been at the forefront developing a range of biological, biotech and pharmaceutical products using scientific resources for nearly five decades now. BSV has influenced patient outcomes in areas such as Women’s Heath, Critical Care and IUI-IVF.
Challenges with its Manual APQR process
Bharat Serums and Vaccines manually conducted its annual product quality review process. This involved manual collection and analysis of data and manual APQR report creation. This practice is prone to errors, could lead to data and document loss and could result in delays in report submission. The other disadvantages include delayed Annual product quality review process, lack of proper authentication and verification, issues with regulatory compliances.
Implementation of AmpleLogic APQR Solution
Bharat Serums and Vaccines is currently using AmpleLogic’s Annual product quality review solution to streamline the process and APQR report generation. As per client feedback, the application is working excellently in the Production Environment.
Auditors have highly appreciated the reports generated through the application. Using AmpleLogic’s Annual product quality review helped Bharat Serums and Vaccines reduce huge cost by implementing CPK PPK analysis within the application using 3 Sigma. The application notifies users about pending actions to be performed through email. Amplelogic provides instant support for Production issues at any point of time with no delay.
IMPLEMENTION IN 10 MONTHS!
The integration of AmpleLogic APQR Software into pharmaceutical companies’ operations marks a significant step towards unlocking business process excellence and ensuring regulatory compliance. By understanding the importance of Annual Product Quality Review (APQR) and using the capabilities, companies can streamline their processes and drive continuous improvement in product quality. With features like data integration and aggregation, automated reporting and analysis, and a risk-based approach, the software enables efficient decision-making and proactive risk management. Additionally, its integration capabilities, cross-functional department collaboration, and compliance management features enhance transparency, efficiency, and regulatory adherence. Through successful implementation, as demonstrated by Bharat Serums and Vaccines, AmpleLogic Annual product quality review Software proves to be a valuable asset in achieving business objectives and ensuring patient safety in the pharmaceutical industry.



























