
Master Batch Record (MBR) is nothing but a comprehensive document that encapsulates detailed instructions for manufacturing a specific product batch. MBR serves as the production process blueprint outlining details such as materials to be used, equipments, procedures and quality controls needed to guarantee product uniformity, safety and efficacy. MBRs are important in maintaining compliance with regulatory requirements as well as ensuring the quality and consistency of pharmaceutical products.
Aims and Objectives of Master Batch Record (MBR)
Standardization: MBRs standardize manufacturing processes for all batches that need to be made. It provides step by step detailed instructions for every stage of the manufacturing lifecycle. This ensure uniformity in product quality across that multiple batches are alike.
Compliance: MBRs must contain written procedures, controls, specifications as required by regulatory authorities like FDA or EMA. This ensures regulatory compliance and helps maintain product quality standards.
Quality Assurance: In addition to critical process parameters defined by various quality control tests and acceptance criteria, MBRs specify how each batch has to processed in order to meet the required standard of quality.
Risk Management: MBRs identify risks related to production processes might lead to product failures at large or deviations and mitigation strategies to minimize the likelihood of product failures or deviations.
Key Components of Master Batch Record
Product Information: Details such as the product name, description, batch number, dosage form and packaging specifications are saved here.
Formulation: The formulation section lists the ingredients, their quantities, and any special instructions for mixing, blending or preparation.
Manufacturing Instructions: Detailed step-by-step instructions for each stage of the manufacturing process, including equipment setup, processing parameters, and in-process controls.
Packaging and Labeling: Instructions for packaging the finished product, including packaging materials, labeling requirements, and batch numbering.
Quality Control Procedures: Specifications for in-process and finished product testing, including sampling plans, test methods, acceptance criteria, and documentation requirements.
Critical Process Parameters (CPPs) and Critical Quality Attributes (CQAs): Identification of key process parameters and quality attributes that must be monitored and controlled to ensure product quality and consistency.
Batch Record Review and Approval: A section for review and approval by designated personnel, confirming that the MBR accurately reflects the intended manufacturing process and meets all regulatory requirements.
Importance of Master Batch Record Management
Document Control: Effective management of MBRs ensures that the most current and approved version of the document is used for each batch, minimizing the risk of errors or deviations.
Workflow Efficiency: Streamlining the approval process for MBRs improves workflow efficiency, allowing for timely production scheduling and execution.
Regulatory Compliance: Maintaining accurate and up-to-date MBRs is crucial for compliance with regulatory requirements, facilitating inspections and audits by regulatory authorities.
Continuous Improvement: Regular review and revision of MBRs enable continuous improvement of manufacturing processes, incorporating lessons learned and best practices.
Types of Batch Records
There are several types of batch records other than Master Batch Record (MBR) that are used in the pharmaceutical industry. For maintaining efficient manufacturing processes and regulatory compliance, understanding the key differences between these records is crucial. Here are some of the common types of batch records:
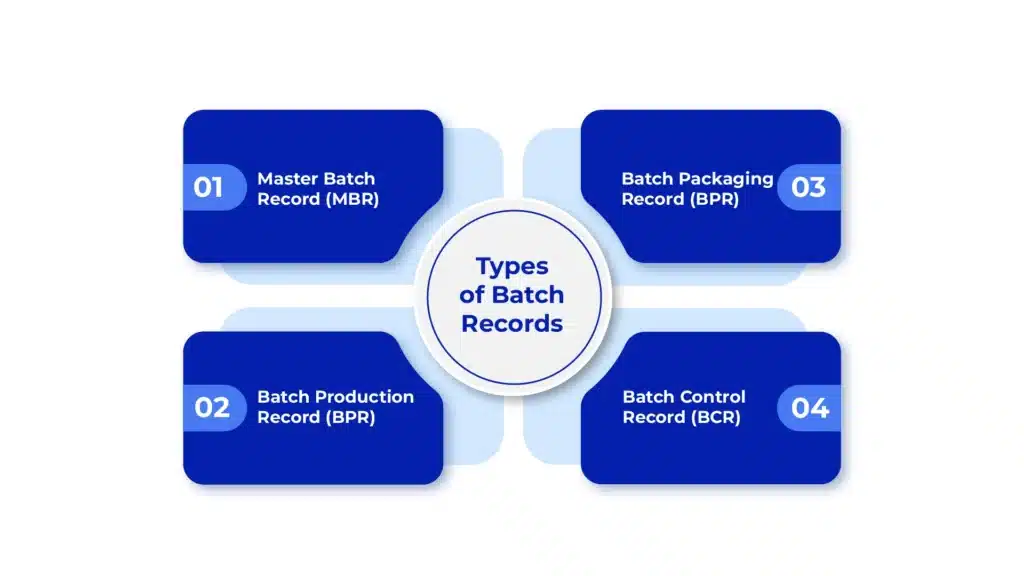
Master Batch Record (MBR): MBRs provide comprehensive instructions for manufacturing specific batches of a product. They serve as a template for the production process, outlining materials, procedures, quality controls, and other critical parameters.
Batch Production Record (BPR): Batch Production Records document actual execution of a manufacturing batch. This includes capturing real-time data such as batch numbers, equipments used, personnel involved, process deviations, and in-process testing results. BPRs ensure that manufacturing activities are conducted in accordance with the approved MBR.
Batch Packaging Record (BPR): Batch Packaging Records detail the packaging and labeling processes for specific product batches. It includes instructions for packaging materials, labeling requirements, batch numbering and any special handling or storage conditions. BPRs ensure compliance with packaging specifications and regulatory requirements.
Batch Control Record (BCR): Batch Control Records provide summary of all activities and controls performed during the manufacturing process. This serves as a comprehensive record of batch-related activities, including material usage, equipment calibration, process parameters, quality control tests, and any deviations or corrective actions taken.
Master Batch Records Vs Batch Records
While Master Batch Record (MBR) is a type of batch record, it’s essential to distinguish between MBRs and other batch records used in pharmaceutical manufacturing:
Master Batch Record (MBR): MBRs serve as the primary document for defining the manufacturing process for a specific product batch. They provide detailed instructions for formulation, processing, packaging, and quality control, serving as a blueprint for consistent and reproducible production.
Batch Records (BPRs, BCRs, etc.): Batch Records, including Batch Production Records (BPRs), Batch Packaging Records (BPRs), and Batch Control Records (BCRs), document the execution of the manufacturing process outlined in the MBR. They provide real-time data and records of activities, controls, and test results performed during batch production and packaging.
cGMP and GAMP 5 Compliance Through MBRs
Master Batch Records (MBRs) align closely with Good Manufacturing Practice (cGMP) regulations and the Good Automated Manufacturing Practice (GAMP) 5 guidelines. Here’s how MBRs support compliance with mandatory regulatory frameworks.
Alignment with cGMP
Documentation and Recordkeeping: MBRs form a key component of documentation in pharmaceutical manufacturing, that is a core principle of cGMP. It provides detailed instructions for each step of the manufacturing process, ensuring consistency, traceability, and accountability.
Quality Assurance: MBRs include specifications for critical process parameters, quality control tests, and acceptance criteria, ensuring that each batch meets the required quality standards. This supports the cGMP principle of ensuring the quality and purity of pharmaceutical products.
Risk Management: MBRs identify potential risks associated with the manufacturing process and provide mitigation strategies to minimize the likelihood of product failures or deviations, aligning with the risk-based approach advocated by cGMP.
Training and Personnel Qualification: MBRs specify the procedures, controls, and responsibilities for each manufacturing step, supporting the training and qualification of personnel involved in production, which is essential for cGMP compliance.
Alignment with GAMP 5 Guidelines
Controlled Documentation: MBRs are considered controlled documents within the GAMP 5 framework, subject to defined change control procedures to ensure that only authorized changes are made and documented appropriately.
Automation and Computerized Systems: In the context of computerized systems used in pharmaceutical manufacturing, MBRs serve as the basis for defining user requirements, functional specifications, and testing protocols, as outlined in GAMP 5.
Data Integrity: MBRs play a crucial role in ensuring data integrity throughout the manufacturing process by documenting all critical manufacturing parameters, quality control tests, and deviations in a complete, accurate, and attributable manner, as required by GAMP 5.
Validation: MBRs are subject to validation activities as part of the validation lifecycle defined by GAMP 5. This includes ensuring that the instructions and controls specified in the MBR are effective in consistently producing pharmaceutical products of the required quality.
Change Control: Changes to MBRs are managed through defined change control procedures, including impact assessment, approval, and documentation of changes, as per the principles outlined in GAMP 5 for managing changes to controlled documents and computerized systems.
How AmpleLogic eBMR can help Manage your Master Batch Records
AmpleLogic’s Electronic Batch Manufacturing Record (eBMR) solution offers robust capabilities for managing Master Batch Records (MBRs) in pharmaceutical manufacturing. It houses features such as version control, automated notifications, real-time reporting, integration with quality management softwares and much more. Here we have highlighted some of the amazing features of AmpleLogic MBR system.
- Centralized Document Management:
Single Platform: AmpleLogic eBMR provides a centralized platform for storing, organizing, and accessing MBRs, ensuring that all stakeholders have access to the latest approved versions.
Version Control: The system maintains version control, tracking revisions and ensuring that only the most current MBRs are used for production.
- Configurable Workflow Automation:
Customizable Workflows: AmpleLogic eBMR allows for the creation of customizable workflows tailored to the specific requirements of each MBR, streamlining the approval process.
Automated Notifications: The system sends automated notifications to relevant personnel when action is required, reducing delays and improving overall efficiency.
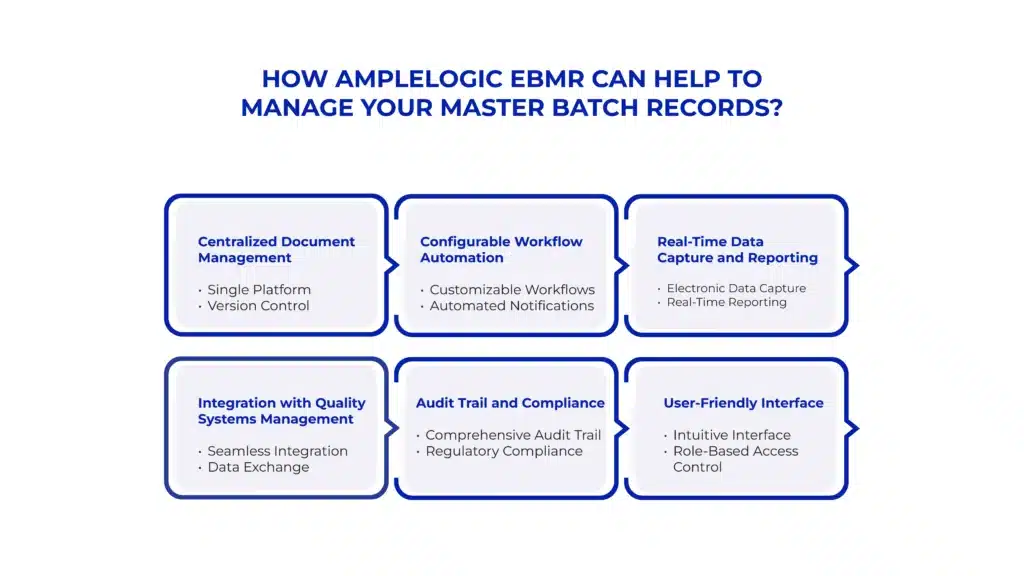
- Real-Time Data Capture and Reporting:
Electronic Data Capture: AmpleLogic eBMR facilitates electronic data capture during the manufacturing process, allowing for real-time recording of production data, deviations, and quality control results.
Real-Time Reporting: The system generates real-time reports on production progress, quality metrics, and compliance status, enabling timely decision-making and proactive problem-solving.
- Integration with Quality Systems:
Seamless Integration: AmpleLogic eBMR integrates seamlessly with quality management systems (QMS), laboratory information management systems (LIMS), and other relevant systems, ensuring data consistency and accuracy across the manufacturing and quality domains.
Data Exchange: The system facilitates automatic data exchange between MBRs and quality systems, enabling efficient management of quality control tests, deviations, and corrective actions.
- Audit Trail and Compliance:
Comprehensive Audit Trail: AmpleLogic eBMR maintains a comprehensive audit trail of all actions taken within the system, providing traceability and accountability for MBR-related activities.
Regulatory Compliance: The system helps ensure regulatory compliance by enforcing standard operating procedures (SOPs), documenting all aspects of the manufacturing process, and facilitating inspection readiness.
- User-Friendly Interface:
Intuitive Interface: AmpleLogic eBMR features a user-friendly interface that is easy to navigate, allowing personnel at all levels to access and interact with MBRs efficiently.
Role-Based Access Control: The system employs role-based access control, ensuring that users only have access to the information and functionality relevant to their roles and responsibilities.
Master Batch Records (MBRs) are essentially documents in the pharmaceutical manufacturing sector that gives detailed instructions for manufacturing of quality and consistent products. AmpleLogic offers advanced software solutions like a BMR system that aligns the requirements of deriving an MBR. It streamlines document management, workflow automation, electronic batch records, integration with quality systems, and audit trail functionality. By utilizing AmpleLogic’s capabilities, pharmaceutical companies can enhance efficiency, compliance and provide excellent quality assurance.



























