
The global pharmaceutical industry has boomed for decades by increasing its market in both the developed and the developing nation. With the evolution of data- and technology-driven changes it has transformed the industry, pharmaceutical companies and being forced to revamp their existing strategies. The emerging markets are becoming more complicated for pharmaceutical companies, making the commercial atmosphere tough and harder for them to sustain. Amidst all the changes, one of the biggest challenges encountered by pharmaceutical players is shifting from their traditional manual log book to digital log book.
Most pharma formulations and chemical manufacturing sites record the details of the operation in a paper-based instrument/equipment usage log book. Depending on the dimension of the organizations, they record information in huge logbooks. In addition, the logbooks maintained by the organizations range from 500-2000, containing multiple product categories.
It is often observed that keeping track and updating the manual logbook and their version is a monotonous job. However, there are higher chances of duplicating the information which can lead to regulatory issues. Let’s go through few challenges encountered by the organization:
Challenges of existing Manual Logbooks in Pharmaceutical Industries
Compliance and regulatory challenges: Maintaining manual logbooks demands immense paperwork and manual entries, which can be prone to human errors and mistakes. Hence leading to compliance and regulatory issues, including fines and penalties.
Productivity and efficiency issues: Manual logbooks can be time-consuming and labour-intensive, demanding much effort and resources to maintain and update. Therefore, the consequence results in productivity and efficiency.
Data accuracy and consistency: It is often maintained by different individuals, utilising different formats and styles, leading to inconsistencies and errors in the data. It can extract meaningful insights and trends from the data complex.
Inventory Management: For pharma companies keeping track of their inventory, meeting constantly changing regulations, supplying promising solutions with the utmost quality, and balancing risks associated with inventory shortages and gluts is very challenging.
Risk Management: Managing risks in drug development and discovery are of extreme importance. Every product and process are exposed to risk, making it important for companies to reduce risks, which can threaten the quality of their products.
Now that we know about the challenges. Let’s see the multiple log book maintained by the manufacturing area followed by engineering, plant maintenance warehouse and more.
To name a few logbooks
- Area cleaning Record
- Preparation & Usage of Disinfectant solution record
- Washing area cleaning Record
- Calibration test record
- Area sequential log
- Equipment/Instrument Sequential Log
While looking at the above example logs, even though the process is the same, organizations still maintain different log formats for each facility due to manually printed logbooks.
According to the experts, out of 1000+ logbooks, nearly 60% of logbooks are required to be recorded daily, remaining weekly, monthly, quarterly and once in 6 months.
Digitalization of LogBooks
The term “digitalization of logbooks” is the game changer for all the organizations using manual logs. It substitutes the traditional ones to eliminate duplication and manipulation. It can only be successful once digitalization of these manual logbook organizations and their technology partners must land on the same page to finalize the scope by harmonizing the existing log books. The objective of this partnership is to eliminate the duplication in the current manual logs. Let’s see the perk of digital logs below:
Real-time monitoring and reporting: Digital log book supply real-time monitoring and reporting of operations, enabling organizations to identify and address issues quickly. It enhances the quality of products and reduces the risk of product recalls.
Data analytics and insights: It offers rich data analytics and insights, enabling organizations to determine trends and patterns in operations, optimize processes, and enhance overall efficiency and productivity.
Collaboration and communication: Fosters collaboration and communication between departments and stakeholders. Hence, they can share information and insights and work together more effectively.
Cost savings: Help organizations save costs by reducing the need for paper-based records, streamlining operations, and improving overall efficiency and productivity.
By addressing these challenges and leveraging the benefits of digital logbook, pharmaceutical organizations can improve their operations, increase productivity and efficiency, and ensure compliance and regulatory compliance.
The road ahead
Looking at the existing diverse logs makes the Project look huge, but nearly 80 harmonized electronic log book can handle nearly 90% of the daily and weekly usage logs.
In case of Organizations with more than 10 sites and multiple product types they can follow the below defined stage wise approach for the Results.
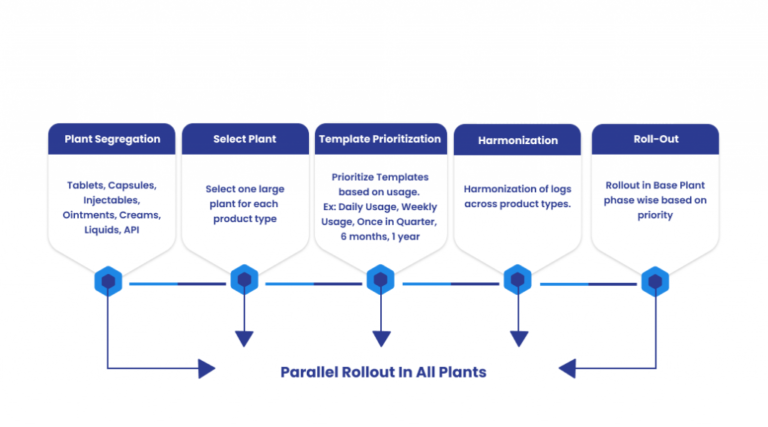
While defining these logs, business users must consider offline logbooks where network connectivity is a big challenge, especially in aseptic areas. In the world of digitalization, it’s high time for your business to step up and go hassle-free to stay ahead in the market.
Author : Manne V Chowdary, Being CEO and Founder at Amplelogic Mr. Manne Associated with 100+ Life science Organizations in creating and executing their Digital Roadmaps.




























