
As the Indian pharma sector embraces digitalization and advanced technologies, several key transformations are expected:
1. Global Competitiveness: By going digital and incorporating Pharma 4.0, Indian pharmaceutical companies can enhance their global competitiveness by being at the forefront of technological innovation and efficiency.
2. Regulatory Compliance: Implementing Pharma 4.0 technologies helps streamline regulatory compliance, making audits and inspections smoother and more efficient.
3. Continuous Manufacturing: Pharma 4.0 facilitates continuous manufacturing processes, enabling seamless production with reduced downtime. This approach increases efficiency and reduces waste.
4. Accelerated Drug Discovery: Advanced computational methods and machine learning algorithms can analyze vast amounts of data, accelerating drug discovery and reducing the time required to bring new drugs to market.
5. Real-time Monitoring and Control: Pharmaceutical companies can monitor manufacturing processes and critical parameters in real-time with IoT and data analytics. This proactive approach allows for early detection of deviations and immediate corrective actions.
To sustain this progress, the industry must focus on several key areas:
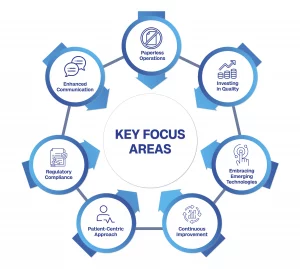
1. Enhanced Communication: Establishing a direct shop floor to higher management connectivity ensures seamless collaboration.
2. Paperless Operations: Minimizing paper usage mitigates data integrity risks and ensures compliance.
3. Investing in Quality: Increasing the quality budget fortifies a commitment to excellence.
4. Embracing Emerging Technologies: Adapting to emerging technologies fosters innovation and efficiency.
5. Continuous Improvement: Pursuing a culture of continuous improvement drives progress and quality enhancement.
6. Patient-Centric Approach: Prioritizing patient needs promotes better healthcare outcomes.
7. Regulatory Compliance: Upholding compliance standards is crucial for sustained growth.
Digital transformation is the key to future success, enabling the Indian pharmaceutical industry to navigate the evolving global landscape. By embracing innovation, fostering regulatory excellence, and investing in technology, the industry can confidently embrace a brighter future.
As we reflect on the future, we invite our readers to share their thoughts on the industry’s challenges and opportunities. The impact of digitalization on drug development, manufacturing, and patient care is an important conversation. We welcome your insights and queries, and together, we can drive the Indian pharmaceutical industry towards a prosperous tomorrow.
–Vijay Patil, Product Manager – AmpleLogic
Empowering the future of pharma through digitalization, innovation and reshaping the processes”



























