
Digitalization: An Evident Automation Revolution, Is automation a step-up for healthcare?
Talking about digitalization will surely uncover a lot of facts, insights and statistics in contemporary industries. Whenever a new technology comes, it comes as a boon to humanity, but it also brings up a lot of unforeseen outcomes, and that’s the whole pact. Similarly, digitalization has impacted many businesses in both ways and the pharma industry is the one among them, especially during the ongoing fitful pandemic.
Life science companies being processed manually from ages are now transforming themselves to adopt digitalization with pharma automation. Yes, it is a big change! Besides, the Food and Drug Administration (FDA) in its 21 CFR Part 11 compliance, has made a regulation that all the paperwork processes have to be made digital with e-documents and e-signatures. The main objective of the regulation is to encourage medical management systems to adopt automation as a pillar to run the entire supply chain smoothly.
Challenges In Healthcare Supply Chain During COVID-19: What are the reasons behind the dysfunctional processes?
With an unpredictable virus-led pandemic on the head, it had been a terrific scenario all around the globe. And healthcare was not an exception, rather it faced or is facing so many challenges in staying stable with the disrupted supply chain system. Lacking automation in pharma, here are some prevalent issues, mentioned below, became hurdles for life science industries:
- Shortage of resources; Raw materials, labours, time
- Coming up with assorted feasible solutions to deal with the virus, as per the demand
- Slow travel time of products (drugs, medicines. etc.) that halted the delivery and reached the market slower
- Staying updated and keeping people updated with the health rules and regulations during the new normal
- Delivering quality assistance with a recovery assurance; Step by step testing, treatment, etc.
- Demand of vaccines all over the world has also been a pressure
- Lacking full-fledged technological involvement in the treatment with minimal automation in pharmaceutical industries
- Disintegration of the whole streamline operation
When we see a lot of challenges in the whole healthcare supply chain, we also have to keep a tab on the deadly repercussions of the pandemic. With a global death of 3.94 million, it is high time to inculcate automation into the life science management systems.
Welcome Digitalization with Low Code Platforms: Why digitalization a tough job for pharma companies?
Where a lot of pharma industries are getting influenced with this idea of digitalization, a few things still are becoming barriers; tough code-run and program-based features.
“But have you heard about Low Code Platforms?”
Yes, that’s what we will be talking about, and that sort of digitalization is the one that will definitely not scare out any pharma business.
Low Code Platform is a more resolved and stress-free version of the app development process. With this, many businesses handle their end-to-end business tasks, starting from customer support to lead generation and many more.
Many pharma automation companies are now adopting Low Code Platforms to accelerate their business as well stay strong in the market, pleasing their target customers.
What do you get with a Low Code Platform to showcase your business in digital mode?
Visual modelling tools (or apps) with in-built features, where you do not have to juggle with confusing codes and programs. You can easily put your information and it will be readable to everyone starting from laypersons to professionals.
- Out-of-the-box functionalities, where you don’t need to start making an app from scratch, rather you can just pick a particular section or module to build.
- Easy drag and drop features make things operate hassle-free for a pharmaceutical automation industry
- An app with pre-installed modules, plug-ins that functions faster
- Accessible to every device
- A fully functional and secured digital platform
- Scalable features allowing further growth possibilities in business
- Transparent application lifecycle management that takes the consent of the user before making any change in the app and also inform the user about everything going on the platform
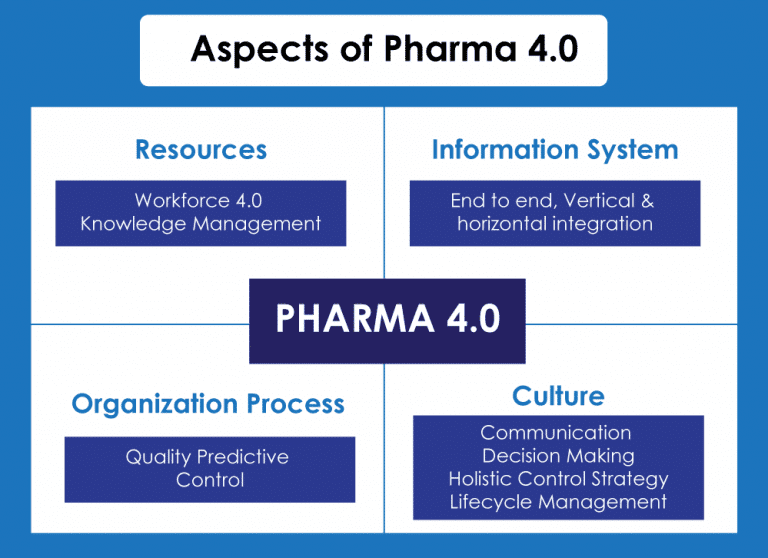
Pharma 4.0: A quick peek: Is digitalization a benefitting deal for pharma sectors?
Of course, digitalization when implemented with all the smart strategies, is the ultimate success code to boost any business. The rapid digitalization in several industries is showing a huge probability of completely rooting out traditional ways of manual processing. And with this, industry 4.0 came into the picture, which is a perfect combination of digital technology and innovation, and it is ready to channelize industrial revolution all around the globe.
So, with industry 4.0 in the pharmaceutical industry we see a good hope of growth in life science industries also, benefiting both the supplier and the consumer. Pharma 4.0 is nothing but a strategic series to be implemented in the pharmaceutical manufacturing process, where every phase of the chain will be digitalized and keenly monitored, starting from the manufacturing operation to the end delivery.
This is an amazing time to accept a profiting transformation. Isn’t it? We at AmpleLogic strive to provide pharmaceutical and life sciences companies with ready-to-use and customizable solutions that can help you transform your business. This will not only make your pharma business smoother but also will help you serve your consumers efficiently. This is certainly a good deal!




























