
In regulated industries such as pharmaceuticals, food and beverages, cosmetics, medical devices, etc adherence to Good Automated Manufacturing Practice (GAMP) standards stands as a non-negotiable pillar for consistent production and product control at the highest quality levels. Central to GMP compliance is the meticulous upkeep of detailed logbooks, serving as the backbone of documentation in manufacturing processes. In this comprehensive exploration, we delve into the indispensable requirements of the GMP Electronic Logbook and their pivotal role in ensuring compliance and upholding product quality.
Requirements for GMP logbooks
Accuracy and Completeness: Logbooks must contain precise and comprehensive records of all relevant activities and processes. This ensures that all data is reliable and can be used for audits, reviews, and compliance checks.
Timeliness: Entries in logbooks should be made in real-time or as close to the actual event as possible to ensure accuracy and reliability of data.
Traceability: Each log entry must be attributable to the person making it, typically through electronic signatures or unique user identifiers. This helps in maintaining accountability and traceability of actions.
Security: Logbooks must be protected against unauthorized access and alterations. Electronic logbooks should have robust security measures, including user authentication and data encryption.
Audit Trails: An essential feature of electronic logbooks is the ability to maintain an audit trail that records all changes and entries, providing a transparent history of all activities.
Compliance with Regulatory Standards: Logbooks must meet the specific requirements set by regulatory bodies such as the FDA, EMA, and others, ensuring that all records support regulatory compliance.
Data Integrity: Ensuring that data is accurate, complete, and maintained in a consistent and reliable manner is crucial. This involves preventing data tampering and ensuring data is retrievable throughout its retention period.
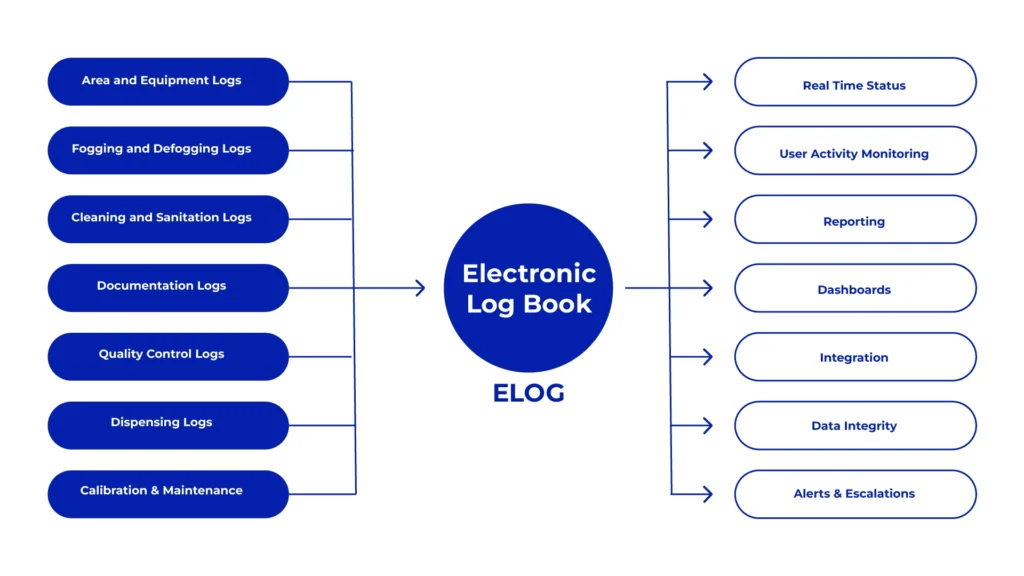
Types of Logs in GMP Compliance
Equipment Utility Logs
Effective equipment management forms a cornerstone in maintaining GMP compliance. Equipment logs serve as repositories for maintenance schedules, cleaning records, calibration logs, and other pertinent information related to machinery used in manufacturing processes. Regular maintenance and calibration bolster the reliability and accuracy of equipment, mitigating the risk of errors or deviations jeopardizing product quality.
Environmental Monitoring
Preserving manufacturing environments to meet specific standards is imperative in preventing contamination and safeguarding product integrity. Logbooks dedicated to environmental monitoring capture data concerning temperature, humidity, cleanliness levels, and other relevant parameters within manufacturing facilities. Monitoring and documenting these conditions enable swift identification and rectification of any deviations that may impact product quality or safety.
Quality Control / Quality Assurance
The cornerstone of GMP compliance lies in quality control. Logbooks serve to record the results of quality control tests and inspections conducted throughout the manufacturing process. These checks validate that products meet predefined specifications and standards, fortifying defenses against defects or deviations that could lead to product recalls or regulatory sanctions.
Material Tracking
From raw materials to finished products, meticulous tracking is essential for GMP compliance. Logbooks facilitate the documentation of material usage, including lot numbers, expiration dates, and quantities employed in each batch. This level of traceability enables swift and precise identification of materials in the event of a recall or quality issue, thereby enhancing safety and consumer confidence.
Cleaning and Sanitization Records
Upholding cleanliness and hygiene in manufacturing facilities is paramount in preventing contamination and ensuring product safety. Logbooks for cleaning and sanitization activities meticulously document procedures, frequencies, and validation records to demonstrate compliance with GMP standards. Thorough documentation of these processes mitigates the risk of cross-contamination and upholds product integrity.
Training Records
Human error poses significant risks in manufacturing operations. Logbooks for training records capture information regarding employee training on GMP procedures and job-specific responsibilities. Ensuring personnel are adequately trained and informed minimizes the likelihood of errors that could compromise product quality or regulatory compliance.
Deviation Reports
During the manufacturing process, deviations from standard operating procedures may inevitably occur. Logbooks for deviation reports meticulously document any encountered deviations alongside investigations and corrective actions implemented. Promptly addressing deviations and implementing corrective measures upholds GMP principles and mitigates risks to product quality and safety.
Supplier Documentation
The quality of raw materials and components sourced from suppliers directly impacts the final product quality. Logbooks for supplier documentation capture information regarding supplier qualifications, material specifications, and audits or inspections conducted at supplier facilities. Thorough documentation ensures accountability and transparency in the supply chain, reducing the risk of substandard inputs affecting product quality.
Complaint Handling
Customer feedback is invaluable in identifying potential issues with products or processes. Logbooks for complaint handling document customer complaints received, alongside investigations and corrective actions taken. Systematically addressing customer concerns and continuously improving processes underscores commitment to customer satisfaction and product quality.
Predictive Analysis
Predictive analysis utilizes eLogbook data in pharmaceuticals for forecasting future trends, optimizing production processes, and enhancing decision-making.
Manufacturing Processes
Electronic logbooks streamline data capture, ensure compliance, and improve efficiency in pharmaceutical manufacturing. They integrate with other systems, facilitate remote collaboration, and provide valuable insights through data analytics, thus digitizing manufacturing processes effectively.
Issuance and retrieval
Electronic logbooks significantly enhance issuance and retrieval processes in pharmaceutical manufacturing and QA by enabling instant issuance, automated approval workflows, version control, quick retrieval, comprehensive audit trails, electronic signatures, and integration with document management systems.
Engineering Logs
Engineering logs meticulously document equipment maintenance, calibration, and environmental monitoring in manufacturing. They ensure regulatory compliance, support audits, and facilitate data-driven decision-making for risk mitigation and efficiency improvement. By tracking maintenance schedules, these logs uphold product quality and regulatory standards, ensuring a reliable manufacturing environment.
In conclusion, maintaining GMP compliance requires the diligent use of detailed and accurate logbooks. By adhering to the outlined requirements and understanding the various types of logs, manufacturers can ensure product quality, safety, and regulatory compliance.



























