
Go Paperless with Document Management System
Gone are the days when we used to keep all our essential information and crucial data in tones of logbooks. Here comes the new era with new innovative options to get those things done without any hassle, Electronic Document Management Software. When you have a lot of data to input, limiting to manual documentation becomes exhausting, expensive, and time-consuming. With this digital add-on, the entire documentation process in contemporary pharma and life sciences industries is managed effortlessly, ensuring the organized creation of automated documents.
By helping several businesses organize their paperwork digitally with DMS, companies are transforming from paper-based to completely paperless systems that are more efficient and easier to access. With numerous helpful features, DMS is becoming essential for all businesses to incorporate into their entire documentation process, promoting further business growth.
Embracing Digital Compliance: The Impact of FDA 21 CFR Part 11 on Documentation in Life Sciences
The FDA 21 CFR part 11 regulation clarifies that all electronic records, including signatures and other documents, are to be treated as final, replacing paper-based records. This signals that contemporary life sciences, pharma, and biotechnology industries should embrace this effective and painless digital trend. With Electronic Document Management, the automatic feeding of data and tracking of necessary information have made the documentation process truly uncomplicated.
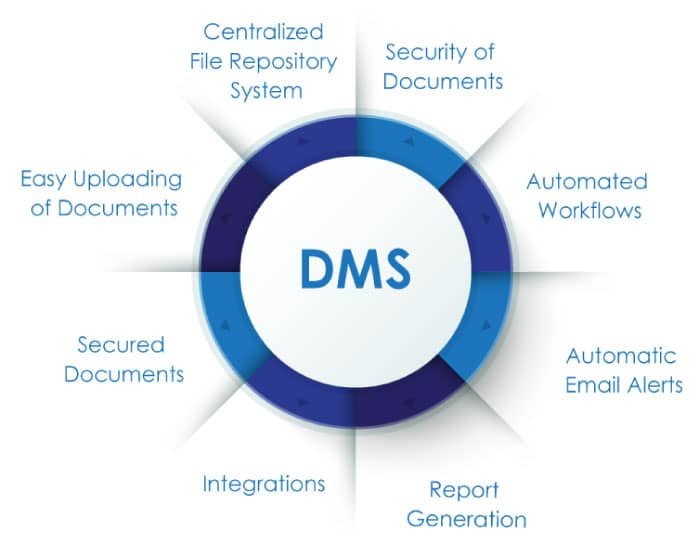
Let us acknowledge this user-friendly innovation and recognize the significant benefits it has bestowed upon pharmaceutical businesses struggling with critical challenges.
- Previously, document creation, review, approval, editing, and rework were all handled manually. However, DMS has streamlined these tasks, managing every stage automatically with minimal human intervention.
- Manual documentation can be time-intensive and disrupt workflows if even one employee is not up to date with the entire process. In contrast, DMS maintains the entire workflow efficiently, saving time and allowing quality time to be invested in other areas of growth.
- In a pharmaceutical or life sciences company, frequent changes in regulations and compliance requirements can be challenging to track with manual documentation. However, with DMS, you can stay updated on all changes and maintain a clear overview of all activities.
- Have you ever wondered where to store those mountains of documents? What will you do when you have an urgent meeting and can’t find the file you need? This is where the DMS application comes in handy. It allows you to manage a central hub for all your important data in one cloud, making it easily accessible whenever needed.
- Manual data entry poses a significant risk of missing important information, incorrect data input, or data mismatches. While these issues are inevitable with manual management, they can be resolved with a DMS application. The software provides instant reminders, notifications, and escalation pop-ups whenever it detects errors or requires data revisions.
- Effective document management goes beyond merely storing paperwork. It facilitates real-time collaboration by providing a centralized platform where interrelated departments, such as production, operations, and quality assurance, can seamlessly share and access documents. This system allows team members to work on the same document simultaneously, with real-time updates ensuring everyone is always on the same page.
- In an organization with many employees, managing their data can be challenging, and missing data can lead to problems. However, with DMS, every detail of all employees is securely stored in a specific cloud and is easily accessible through secure, personalized credentials.
- Using DMS to store data, rather than relying on manual documentation, ensures that data is safe from destruction due to natural calamities or fires in the storage area.
As businesses increasingly shift from human-operated to automated processes, AmpleLogic’s Document Management System is helping both budding and established pharma and life sciences companies expand their digitalization efforts with a modern touch. Its mission is to drive end-to-end digitalization across the pharma industry, leveraging the impact of Pharma 4.0 and full-fledged automation. Schedule a call with the AmpleLogic team to enroll for a free demonstration of their Electronic Document Management System Software.




























